Exam 6: An Overview of Organic Reactions
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
Instructions: Add curved arrows to the following reaction(s)to indicate the flow of electrons in each.
-Indicate flow: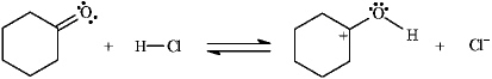
(Essay)
4.8/5  (34)
(34)
Instructions: The reaction below is commonly used as a laboratory preparation of cyclohexene.Use this reaction to answer the following question(s). 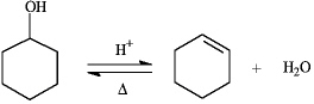 -Refer to instructions.If this reaction under a given set of conditions has a Keq value of 5.67,what percent conversion to the product would be expected?
-Refer to instructions.If this reaction under a given set of conditions has a Keq value of 5.67,what percent conversion to the product would be expected?
(Multiple Choice)
4.8/5  (50)
(50)
Instructions: Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s). 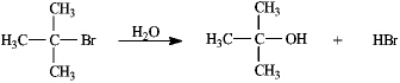 -Refer to instructions.This reaction is an example of:
-Refer to instructions.This reaction is an example of:
(Multiple Choice)
4.7/5  (34)
(34)
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows.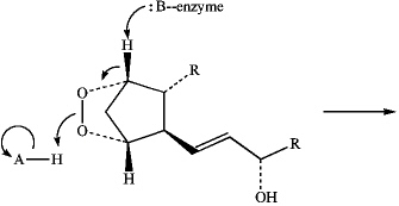
(Essay)
4.9/5  (44)
(44)
Instructions: Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
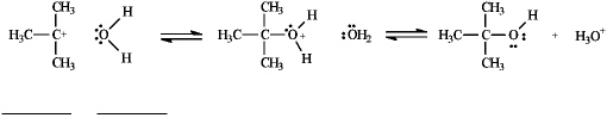 -Refer to instructions.Draw arrows on the structures above showing electron flow in steps two and three of this reaction.
-Refer to instructions.Draw arrows on the structures above showing electron flow in steps two and three of this reaction.
(Essay)
4.8/5  (33)
(33)
Instructions: The reaction below is commonly used as a laboratory preparation of cyclohexene.Use this reaction to answer the following question(s). 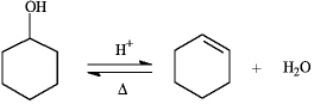 -Refer to instructions.The forward and reverse reactions are classified,respectively,as:
-Refer to instructions.The forward and reverse reactions are classified,respectively,as:
(Multiple Choice)
4.8/5  (42)
(42)
In the following generic reaction between a halogen (X)and an alkane (R),which of the following steps would be considered a chain termination step?
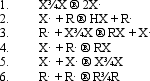
(Short Answer)
4.9/5  (31)
(31)
Instructions: Classify each structure below as a nucleophile or electrophile,and briefly explain your choice.
-Classify and explain:
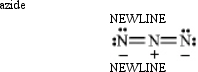
(Essay)
4.7/5  (34)
(34)
In theory,upon reaction with water in the presence of a strong acid,which of the following will produce more than one isomeric product?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 32 of 32
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)