Exam 6: An Overview of Organic Reactions
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
Instructions: Classify each structure below as a nucleophile or electrophile,and briefly explain your choice.
-Classify and explain:
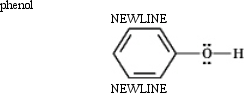
Free
(Essay)
4.8/5  (40)
(40)
Correct Answer:
Phenol can be a nucleophile or an electrophile 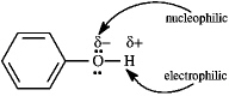
In an organic reaction,which of the following is most likely to function as only a nucleophile?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
D
How many monochlorosubstitution products are possible for 2,3-dimethylbutane?
Free
(Multiple Choice)
5.0/5  (36)
(36)
Correct Answer:
B
Instructions: Use the first step of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s). 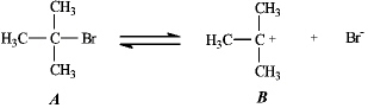 -Refer to instructions.Species B is:
-Refer to instructions.Species B is:
(Multiple Choice)
5.0/5  (33)
(33)
Consider the following reaction:
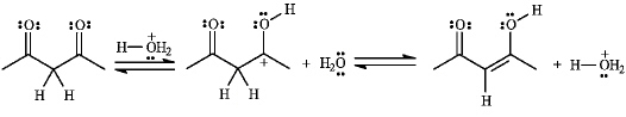 a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b) If the second step of the reaction were the slow step, briefly explain how the values of
a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b) If the second step of the reaction were the slow step, briefly explain how the values of 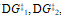 , and DG° would change.
, and DG° would change.
(Essay)
4.7/5  (40)
(40)
Instructions: Add curved arrows to the following reaction(s)to indicate the flow of electrons in each.
-Indicate flow: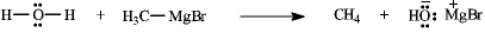
(Essay)
4.8/5  (37)
(37)
Instructions: Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
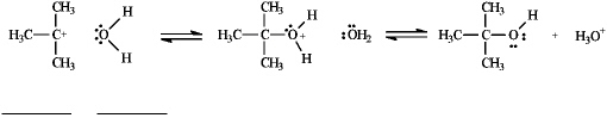 -Refer to instructions.Label the nucleophile,Nu,and the electrophile,E+,in the blanks provided under the structures.
-Refer to instructions.Label the nucleophile,Nu,and the electrophile,E+,in the blanks provided under the structures.
(Essay)
4.8/5  (44)
(44)
Instructions: Use the first step of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
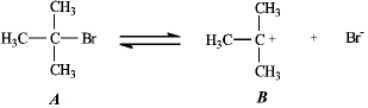 -Refer to instructions.Add curved arrows to indicate electron flow in the first step.
-Refer to instructions.Add curved arrows to indicate electron flow in the first step.
(Essay)
4.9/5  (39)
(39)
The structures below show the stepwise bond making and bond breaking in this reaction.Draw curved arrows to show the electron flow that has occurred in each step.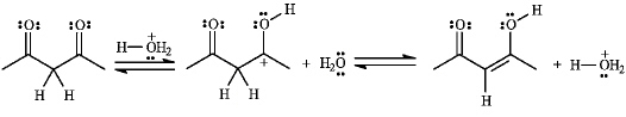
(Essay)
4.9/5  (31)
(31)
Instructions: Identify the functional groups present in each compound below,and predict the direction of polarity in each.
-Identify and predict:
mustard gas Cl-CH2CH2-S-CH2CH2-Cl
(Essay)
4.9/5  (38)
(38)
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
-Identify and label: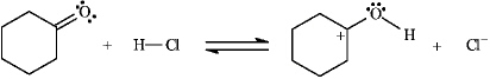
(Essay)
4.9/5  (41)
(41)
Predict the two alcohol addition products obtained by reaction of the following alkene with aqueous acid.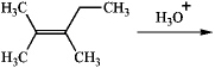
(Essay)
4.8/5  (28)
(28)
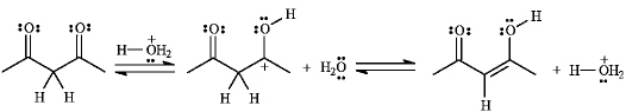
If the yield for the following reaction is 76%,calculate Keq and predict the sign of DG
.
(Essay)
4.8/5  (42)
(42)
Instructions: Identify the functional groups present in each compound below,and predict the direction of polarity in each.
-Identify and predict:
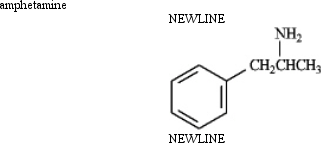
(Essay)
4.8/5  (42)
(42)
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
-Identify and label:
(Essay)
4.8/5  (47)
(47)
Which of the following is a characteristic of a polar reaction?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following correctly compares the two elements in terms of polarizability?
(Multiple Choice)
4.8/5  (38)
(38)
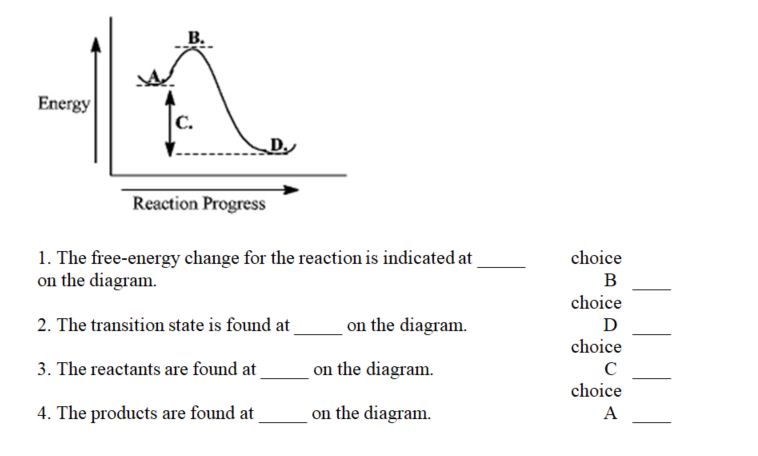
Instructions: Use the reaction energy diagram below to answer the following question(s).
-
(Essay)
4.8/5  (37)
(37)
In the following generic reaction between a halogen (X)and an alkane (R),which of the following steps occurs slowly due to low concentration of reactants?
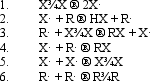
(Short Answer)
4.8/5  (38)
(38)
Showing 1 - 20 of 32
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)