Exam 2: Polar Covalent Bonds: Acids and Bases
Exam 1: Structure and Bonding30 Questions
Exam 2: Polar Covalent Bonds: Acids and Bases35 Questions
Exam 3: Organic Compounds: Alkanes and Their Stereochemistry32 Questions
Exam 4: Organic Compounds: Cycloalkanes and Their Stereochemistry18 Questions
Exam 5: Stereochemistry at Tetrahedral Centers33 Questions
Exam 6: An Overview of Organic Reactions32 Questions
Exam 7: Alkenes and Alkynes34 Questions
Exam 8: Reactions of Alkenes and Alkynes37 Questions
Exam 9: Aromatic Compounds36 Questions
Exam 10: Structure Determination: Mass Spectrometry,infrared Spectroscopy,and Ultraviolet Spectroscopy41 Questions
Exam 11: Structure Determination: Nuclear Magnetic Resonance Spectroscopy37 Questions
Exam 12: Organohalides: Nucleophilic Substitutions and Eliminations37 Questions
Exam 13: Alcohols,phenols,and Thiols: Ethers and Sulfides19 Questions
Exam 14: Aldehydes and Ketones: Nucleophilic Addition Reactions30 Questions
Exam 15: Carboxylic Acids and Nitriles23 Questions
Exam 16: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions42 Questions
Exam 17: Carbonyl Alpha-Substitution and Condensation Reactions84 Questions
Exam 18: Amines and Heterocycles28 Questions
Exam 19: Biomolecules: Amino Acids,peptides,and Proteins36 Questions
Exam 20: Amino Acid Metabolism34 Questions
Exam 21: Biomolecules: Carbohydrates42 Questions
Exam 22: Carbohydrate Metabolism41 Questions
Exam 23: Biomolecules: Lipids and Their Metabolism31 Questions
Exam 24: Biomolecules: Nucleic Acids and Their Metabolism23 Questions
Select questions type
Rank the following in order of decreasing importance as a contributing resonance structure to the molecular structure of acetone,CH3COCH3 (more important > less important). 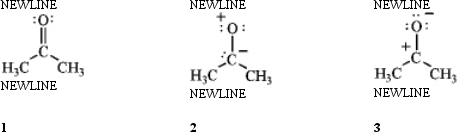
(Multiple Choice)
4.8/5  (41)
(41)
Instructions: Consider the molecules below to answer the following question. 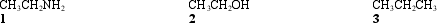 -Refer to instructions.Which of the following is an accurate description of the noncovalent interactions between like molecules?
-Refer to instructions.Which of the following is an accurate description of the noncovalent interactions between like molecules?
(Multiple Choice)
4.9/5  (47)
(47)
Write an equation for the reaction of boron trifluoride,an important reagent in organic chemistry,with trimethylamine.Represent the movement of electrons with a curved arrow,and show the formal charges on the atoms in the product.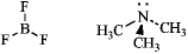
(Essay)
4.8/5  (45)
(45)
Instructions: Label the acid and base in each reaction below.
-Label:
(Essay)
4.9/5  (36)
(36)
Instructions: Refer to the following equation to answer the question(s)below.
 -Refer to instructions.The strongest Brønsted-Lowry base in the equation is indicated by letter _____.
-Refer to instructions.The strongest Brønsted-Lowry base in the equation is indicated by letter _____.
(Multiple Choice)
4.9/5  (33)
(33)
Instructions: Consider the reaction below to answer the following question.
 -Refer to instructions.Using the curved arrow formalism,show the flow of electrons for this reaction.
-Refer to instructions.Using the curved arrow formalism,show the flow of electrons for this reaction.
(Essay)
4.8/5  (24)
(24)
Identify the reactants and product in the reaction below as acids or bases and specify whether they are Lewis and/or Brønsted-Lowry.
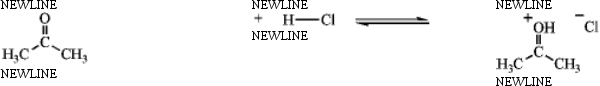
(Essay)
4.7/5  (32)
(32)
Write an equation for the reaction of the alkaloid ephedrine with a proton,showing the structure of its conjugate base.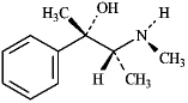
(Essay)
4.8/5  (41)
(41)
Which of the following statements is not true regarding resonance structures?
(Multiple Choice)
4.7/5  (42)
(42)
Instructions: Refer to the following equation to answer the question(s)below.  -Refer to instructions.Will this reaction take place as written in the forward direction? Explain.
-Refer to instructions.Will this reaction take place as written in the forward direction? Explain.
(Essay)
4.8/5  (28)
(28)
Consider the structure of acetic acid shown below. 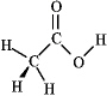 In the electrostatic potential map of acetic acid,in which of the following bonds would the terminal atom appear as the deepest shade of red?
In the electrostatic potential map of acetic acid,in which of the following bonds would the terminal atom appear as the deepest shade of red?
(Multiple Choice)
4.8/5  (41)
(41)
Guanidine is a fairly strong amine base that is attached to the amino acid arginine.Draw three resonance forms for the conjugate acid of guanidine.
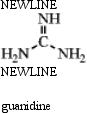
(Essay)
4.9/5  (33)
(33)
The following shows an intermediate used in a Grignard synthesis.Which atom will inductively donate electrons in this species? 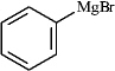
(Multiple Choice)
4.8/5  (40)
(40)
Use the curved arrow method to show the electron movement,and label the acid,base,conjugate acid,and conjugate base.
(Essay)
4.9/5  (30)
(30)
Showing 21 - 35 of 35
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)