Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemistry: The Study of Change153 Questions
Exam 2: Atoms, Molecules, and Ions141 Questions
Exam 3: Mass Relationships in Chemical Reactions168 Questions
Exam 4: Reactions in Aqueous Solution161 Questions
Exam 5: Gases109 Questions
Exam 6: Thermo-Chemistry111 Questions
Exam 7: Quantum Theory and the Electronic Structure of Atoms115 Questions
Exam 8: Periodic Relationships Among the Elements119 Questions
Exam 9: Chemical Bonding I: Basic Concepts118 Questions
Exam 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals122 Questions
Exam 11: Intermolecular Forces and Liquids and Solids140 Questions
Exam 12: Physical Properties of Solutions109 Questions
Exam 13: Chemical Kinetics114 Questions
Exam 14: Chemical Equilibrium100 Questions
Exam 15: Acids and Bases163 Questions
Exam 16: Acid-Base Equilibria and Solubility Equilibria110 Questions
Exam 17: Chemistry in the Atmosphere41 Questions
Exam 18: Entropy, Free Energy, and Equilibrium112 Questions
Exam 19: Electrochemistry138 Questions
Exam 20: Metallurgy and the Chemistry of Metals58 Questions
Exam 21: Nonmetallic Elements and Their Compounds41 Questions
Exam 22: Transition Metal Chemistry and Coordination Compounds80 Questions
Exam 23: Nuclear Chemistry112 Questions
Exam 24: Organic Chemistry57 Questions
Exam 25: Synthetic and Natural Organic Polymers42 Questions
Select questions type
A chemistry student determined the empirical formula for tungsten oxide (WxOy).To do so, he heated tungsten with oxygen in a crucible.The data that he recorded are shown below: 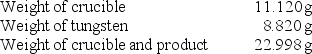 What is the empirical formula of tungsten oxide?
What is the empirical formula of tungsten oxide?
(Short Answer)
4.7/5  (30)
(30)
How many moles of iron are present in an iron cylinder that weighs 25 g?
(Short Answer)
4.9/5  (40)
(40)
Common gases used in laboratories are generally obtained from pressurized metal gas cylinders, but for small amounts of occasionally-used gases, it is sometimes easier just to prepare them chemically.For example, oxygen gas can be prepared by heating KMnO4(s)according to the following chemical reaction:
2KMnO4(s) K2MnO4(s)+ MnO2(s)+ O2(g)
How many grams of KMnO4 would you need to produce 0.27 moles of O2, assuming 100% conversion? The molar mass of KMnO4 is 158.034 g/mol.
(Short Answer)
4.9/5  (36)
(36)
What mass of sodium nitrate would be produced from the complete reaction of 1.00 mol of lead nitrate?
2 NaCl + Pb(NO3 )2 2 NaNO3 + PbCl2
(Short Answer)
4.8/5  (34)
(34)
The element oxygen consists of three naturally occuring isotopes: 16O, 17O, and 18O.The atomic mass of oxygen is 16.0 amu. What can be implied about the relative abundances of these isotopes?
(Multiple Choice)
4.9/5  (35)
(35)
When 22.0 g NaCl and 21.0 g H2SO4 are mixed and react according to the equation below, which is the limiting reagent?
2NaCl + H2SO4 Na2SO4 + 2HCl
(Multiple Choice)
4.9/5  (35)
(35)
Washing soda is a hydrate of sodium carbonate.Elemental analysis of a sample of washing soda gave 4.20% C and 7.05% H.What is the formula for washing soda?
(Multiple Choice)
4.8/5  (40)
(40)
How many moles of aluminum are present in an Al cylinder with a mass of 15 g?
(Short Answer)
4.7/5  (38)
(38)
Balance the following equation using the smallest set of whole numbers, then add together the coefficients.Do not forget to count coefficients of one.The sum of the coefficients is ___ CH4 + ___ Cl2 ___ CCl4 + ___ HCl
(Multiple Choice)
4.8/5  (39)
(39)
Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 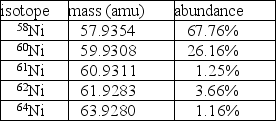 A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
(Essay)
4.9/5  (41)
(41)
What is the theoretical yield of PI3 if 48.0 g of I2 are reacted with an excess of phosphorus according to the following chemical equation?
2P(s)+ 3I2(s) 2PI3(s)
(Short Answer)
4.9/5  (37)
(37)
Balance the following equation using the smallest set of whole numbers, then add together the coefficients.Do not forget to count coefficients of one.The sum of the coefficients is ___ Al + ___ H2SO4 ___ Al2(SO4)3 + ___ H2
(Multiple Choice)
4.9/5  (36)
(36)
Acetylene gas, HCCH(g), can be generated in the laboratory by adding calcium carbide to excess water, as shown in the following reaction
CaC2(s)+ H2O(l) HCCH(g)+ CaO(s)
How many grams of CaC2 would be required to generate 0.20 moles of HCCH(g)?
(Short Answer)
4.9/5  (36)
(36)
Showing 41 - 60 of 168
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)