Exam 3: Elements of Chemistry
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which of these properties describes a metal?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
A sample of water that is 99.9999 percent pure contains 0.0001 percent impurities. Consider from Chapter 1 that a glass of water contains on the order of a trillion trillion (1 × 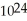 )molecules. If 0.0001 percent of these molecules were the molecules of some impurity, about how many impurity molecules would this be?
)molecules. If 0.0001 percent of these molecules were the molecules of some impurity, about how many impurity molecules would this be?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
In the winter Vermonters make a tasty treat called "sugar on snow" in which they pour boiled-down maple syrup onto a scoop of clean fresh snow. As the syrup hits the snow it forms a delicious taffy. Which of the following changes are involved in the making of sugar on snow?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following images could describe an element at the atomic level?
(Multiple Choice)
4.8/5  (42)
(42)
How would you classify the following material? a cappuccino (with foam)
(Multiple Choice)
4.9/5  (42)
(42)
Compared to microtechnology, nanotechnology focuses on a scale that is about ________.
(Multiple Choice)
4.9/5  (43)
(43)
Classify the following as element, compound, or mixture, and justify your classifications: sodium chloride, stainless steel, sugar, aluminum, ice.
(Multiple Choice)
4.8/5  (38)
(38)
Someone argues that he or she doesn't drink tap water because it contains thousands of molecules of some impurity in each glass. How would you respond in defense of the water's purity, if it indeed does contain thousands of molecules of some impurity per glass?
(Multiple Choice)
4.9/5  (36)
(36)
Is the air in your house a homogeneous or heterogeneous mixture?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following would be considered a homogeneous mixture?
(Multiple Choice)
4.9/5  (34)
(34)
Which chemical family is composed almost entirely of synthetic elements?
(Multiple Choice)
4.9/5  (53)
(53)
Helium, He, is a nonmetallic gas and the second element in the periodic table. Rather than being placed adjacent to hydrogen, H, however, helium is placed on the far right of the table because ________.
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)