Exam 3: Elements of Chemistry
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
The systematic names for water, ammonia, and methane are dihydrogen monoxide, 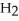 O; trihydrogen nitride, N
O; trihydrogen nitride, N 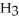 ; and tetrahydrogen carbide, C
; and tetrahydrogen carbide, C 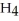 . Why do most people, including chemists, prefer to use the common names for these compounds?
. Why do most people, including chemists, prefer to use the common names for these compounds?
(Multiple Choice)
4.8/5  (35)
(35)
Which element would have chemical properties the most similar to chlorine (Cl)?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following would be considered a heterogeneous mixture?
(Multiple Choice)
4.9/5  (36)
(36)
What happens to the properties of elements across any period of the periodic table?
(Multiple Choice)
4.8/5  (41)
(41)
Which of these does not describe a metal at room temperature?
(Multiple Choice)
4.8/5  (32)
(32)
If you have one molecule of CO2, how many molecules of O2 does it contain?
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following would not be considered a chemical property?
(Multiple Choice)
4.7/5  (35)
(35)
How would you classify the following material? swimming pool water
(Multiple Choice)
4.8/5  (44)
(44)
A sample of iron weighs more after it rusts because ________.
(Multiple Choice)
4.8/5  (47)
(47)
The Colorado River water in Colorado has a salinity of about 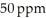 . By the time this water passes into Mexico its salinity has increased to about
. By the time this water passes into Mexico its salinity has increased to about 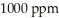 . How many milligrams of salts have been added to each liter of water?
. How many milligrams of salts have been added to each liter of water?
(Multiple Choice)
4.8/5  (38)
(38)
How would you classify the following material? coffee (with milk)
(Multiple Choice)
4.8/5  (41)
(41)
Some products on the market today involving nanotechnology include ________.
(Multiple Choice)
4.8/5  (39)
(39)
Showing 81 - 100 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)