Exam 13: Properties of Solutions
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The concentration of HCl in a solution that is prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ molal.
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the mole fraction of HCl in a 7.20% (by mass)aqueous solution.
(Multiple Choice)
4.7/5  (28)
(28)
Calculate the molarity of phosphoric acid (H3PO4)in a 38.4% (by mass)aqueous solution.
(Multiple Choice)
4.9/5  (39)
(39)
What is the freezing point (°C)of a solution prepared by dissolving 11.3 g of Ca(NO3)2 (formula weight = 164 g/mol)in 115 g of water? The molal freezing point depression constant for water is 1.86 °C/m.
(Multiple Choice)
4.8/5  (44)
(44)
A solution contains 150.8 grams of NaCl in 678.3 grams of water. Calculate the vapor pressure lowering (in torr)of the solution at 25.0 °C. (Note: The vapor pressure of pure water at 25.0 °C is 23.76 torr.)
(Short Answer)
4.9/5  (30)
(30)
A solution is prepared by dissolving 15.0 g of NH3 in 250.0 g of water. The density of the resulting solution is 0.974 g/mL. The molality of NH3 in the solution is ________ m.
(Multiple Choice)
4.8/5  (45)
(45)
Calculate the molality of a 10.0% (by mass)aqueous solution of hydrochloric acid.
(Multiple Choice)
4.7/5  (30)
(30)
A solution is prepared by dissolving calcium chloride in water and diluting to 500.0 mL. If this solution contains 44 ppm chloride ions, the concentration of calcium ions is ________ ppm.
(Multiple Choice)
4.7/5  (42)
(42)
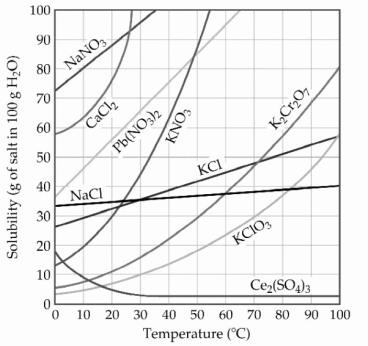 -A sample of potassium chlorate (15.0 g)is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
-A sample of potassium chlorate (15.0 g)is dissolved in 201 g of water at 70 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the molality of a 27.0% (by mass)aqueous solution of nitric acid.
(Multiple Choice)
5.0/5  (37)
(37)
Which of the following substances is more likely to dissolve in water?
(Multiple Choice)
4.9/5  (38)
(38)
A solution contains 30 ppm of benzene. The density of the solution is 1.00 g/mL. This means that ________.
(Multiple Choice)
4.9/5  (33)
(33)
 -A sample of potassium nitrate (49.0 g)is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and a small amount of precipitate is observed. This solution is ________.
-A sample of potassium nitrate (49.0 g)is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and a small amount of precipitate is observed. This solution is ________.
(Multiple Choice)
4.8/5  (38)
(38)
The concentration of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 16 g of urea in 39 g of H2O is ________ molal.
(Multiple Choice)
4.9/5  (42)
(42)
A solution is prepared by dissolving 6.00 g of an unknown nonelectrolyte in enough water to make 1.00 L of solution. The osmotic pressure of this solution is 0.750 atm at 25.0 °C. What is the molecular weight (g/mol)of the unknown solute?
(Multiple Choice)
4.9/5  (37)
(37)
A solution is prepared by adding 1.10 mol of KCl to 889 g of water. The concentration of KCl is ________ molal.
(Multiple Choice)
4.9/5  (43)
(43)
A solution is prepared by dissolving 2.60 g of a strong electrolyte (formula weight = 101 g/mol)in enough water to make 1.00 L of solution. The osmotic pressure of the solution is 1.25 atm at 25.0 °C. What is the van't Hoff factor (i)for the unknown solute?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following liquids will have the lowest freezing point?
(Multiple Choice)
4.7/5  (34)
(34)
The value of the boiling-point-elevation constant (Kb)depends on the identity of the solvent.
(True/False)
4.9/5  (35)
(35)
Showing 141 - 160 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)