Exam 13: Properties of Solutions
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Of the following, a 0.2 M aqueous solution of ________ will have the highest freezing point.
(Multiple Choice)
4.8/5  (30)
(30)
Which one of the following substances would be the most soluble in CCl4?
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the freezing point of a 0.05500 m aqueous solution of NaNO3. The molal freezing-point-depression constant of water is 1.86 °C/m.
(Multiple Choice)
4.8/5  (34)
(34)
Of the concentration units below, only ________ uses kg of solvent in its calculation.
(Multiple Choice)
4.7/5  (34)
(34)
A solution contains 150.8 grams of NaCl in 678.3 grams of water. Calculate the vapor pressure of water (in torr)over the solution at 25.0 °C. (Note: The vapor pressure of pure water at 25.0 °C is 23.76 torr.)
(Short Answer)
4.8/5  (36)
(36)
The process of solute particles being surrounded by solvent particles is known as ________.
(Multiple Choice)
4.7/5  (38)
(38)
A solution is prepared by dissolving 2.00 g of glycerin (C3H8O3)in 201 g of ethanol (C2H5OH). The freezing point of the solution is ________°C. The freezing point of pure ethanol is -114.6 °C at 1 atm. The molal-freezing-point-depression constant (Kf)for ethanol is 1.99 °C/m. The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol, respectively.
(Multiple Choice)
4.8/5  (29)
(29)
Which one of the following substances would be the least soluble in CCl4?
(Multiple Choice)
4.8/5  (30)
(30)
What is the molarity of sodium chloride in solution that is 13.0% by mass sodium chloride and that has a density of 1.10 g/mL?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following substances is more likely to dissolve in CH3OH?
(Multiple Choice)
5.0/5  (36)
(36)
A solution is prepared by adding 40.00 g of lactose (milk sugar)to 110.0 g of water at 55 °C The partial pressure of water above the solution is ________ torr. The vapor pressure of pure water at 55 °C is 118.0 torr. The MW of lactose is 342.3 g/mol.
(Multiple Choice)
4.8/5  (38)
(38)
The concentration (M)of HCl in a solution prepared by dissolving 5.5 g of HCl in 200 g of C2H6O is ________ M. The density of the solution is 0.79 g/mL.
(Multiple Choice)
4.7/5  (47)
(47)
The vapor pressure of pure water at 25 °C is 23.8 torr. What is the vapor pressure (torr)of water above a solution prepared by dissolving 18.0 g of glucose (a nonelectrolyte, MW = 180.0 g/mol)in 95.0 g of water?
(Multiple Choice)
4.8/5  (44)
(44)
Calculate the molality of a 10.0% (by mass)aqueous solution of hydrochloric acid.
(Multiple Choice)
4.8/5  (38)
(38)
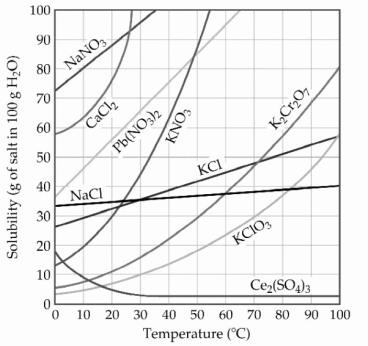 -A sample of potassium nitrate (49.0 g)is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
-A sample of potassium nitrate (49.0 g)is dissolved in 101 g of water at 100 °C, with precautions taken to avoid evaporation of any water. The solution is cooled to 30.0 °C and no precipitate is observed. This solution is ________.
(Multiple Choice)
4.8/5  (31)
(31)
As the concentration of a solute in a solution increases, the freezing point of the solution ________ and the vapor pressure of the solution ________.
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following will have an ideal van't Hoff factor (i)value of 1?
(Multiple Choice)
4.7/5  (34)
(34)
What is the molality of sodium chloride in solution that is 13.0% by mass sodium chloride and that has a density of 1.10 g/mL?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 41 - 60 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)