Exam 17: Additional Aspects of Aqueous Equilibria
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The addition of hydrochloric acid and ________ to water produces a buffer solution.
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the pH of a buffer solution that contains 0.820 grams of sodium acetate and 0.010 moles of acetic acid in 100 ml of water. The Ka of acetic acid is 1.77 × 10-5.
(Short Answer)
4.8/5  (32)
(32)
What is the molar solubility of silver carbonate (Ag2CO3)in water? The solubility-product constant for Ag2CO3 is 8.1 × 10-12 at 25 °C.
(Multiple Choice)
4.7/5  (45)
(45)
The solubility of lead (II)chloride (PbCl2)is 1.6 × 10-2 M. What is the Ksp of PbCl2?
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the pH of a solution prepared by dissolving 0.270 mol of formic acid (HCO2H)and 0.260 mol of sodium formate (NaCO2H)in water sufficient to yield 1.00 L of solution. The Ka of formic acid is 1.77 × 10-4.
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the percent ionization of formic acid (HCO2H)in a solution that is 0.152 M in formic acid. The Ka of formic acid is 1.77 × 10-4.
(Multiple Choice)
4.8/5  (42)
(42)
Suppose you have just added 100.0 ml of a solution containing 0.5000 moles of acetic acid per liter to 400.0 ml of 0.5000 M NaOH. What is the final pH? The Ka of acetic acid is 1.77 × 10-5.
(Short Answer)
4.7/5  (36)
(36)
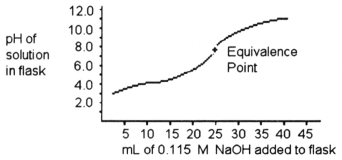 -A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The concentration of the monoprotic acid is about ________ mol/L.
-A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The concentration of the monoprotic acid is about ________ mol/L.
(Multiple Choice)
4.8/5  (44)
(44)
What is the pH of a buffer solution that is 0.172 M in hypochlorous acid (HClO)and 0.131 M in sodium hypochlorite? The Ka of hypochlorous acid is 3.8 × 10-8.
(Multiple Choice)
4.9/5  (46)
(46)
Which of the following could be added to a solution of potassium fluoride to prepare a buffer?
(Multiple Choice)
4.8/5  (36)
(36)
The pH of a solution prepared by mixing 50.0 mL of 0.125 M KOH and 50.0 mL of 0.125 M HCl is ________.
(Multiple Choice)
4.8/5  (39)
(39)
A solution of NaF is added dropwise to a solution that is 0.0122 M in Ba2+. When the concentration of F- exceeds ________ M, BaF2 will precipitate. Neglect volume changes. For BaF, Ksp = 1.7 × 10-6.
(Multiple Choice)
4.9/5  (38)
(38)
A buffer solution with a pH of 4.31 is prepared with 1.0 M HC2H3O2 and ________ M NaC2H3O2. The Ka of HC2H3O2 is 1.8 × 10-5.
(Multiple Choice)
4.9/5  (37)
(37)
In which of the following aqueous solutions would you expect AgF to have the highest solubility?
(Multiple Choice)
4.8/5  (39)
(39)
A 50.0 mL sample of an aqueous H2SO4 solution is titrated with a 0.375 M NaOH solution. The equivalence point is reached with 62.5 mL of the base. The concentration of H2SO4 is ________ M.
(Multiple Choice)
4.9/5  (37)
(37)
Which below best describe(s)the behavior of an amphoteric hydroxide in water?
(Multiple Choice)
4.8/5  (29)
(29)
Showing 21 - 40 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)