Exam 17: Additional Aspects of Aqueous Equilibria
Exam 1: Introduction: Matter and Measurement163 Questions
Exam 2: Atoms, Molecules, and Ions250 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations178 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry180 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding147 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry114 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
A 25.0-mL sample of 0.150 M hydrocyanic acid is titrated with a 0.150 M NaOH solution. What is the pH before any base is added? The Ka of hydrocyanic acid is 4.9 × 10-10.
(Multiple Choice)
4.8/5  (38)
(38)
________ analysis determines only the presence or absence of a particular metal ion, whereas ________ analysis determines how much of a given substance is present.
(Short Answer)
4.7/5  (33)
(33)
A buffer solution with a pH of 4.63 is prepared with 0.14 M formic acid and ________ M sodium formate. The Ka of formic acid is 1.8 × 10-4.
(Multiple Choice)
4.9/5  (42)
(42)
A 25.0 mL sample of 0.723 M HClO4 is titrated with a 0.273 M KOH solution. The H3O+ concentration after the addition of 0.00 mL of KOH is ________ M.
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following could be added to a solution of acetic acid to prepare a buffer?
(Multiple Choice)
5.0/5  (37)
(37)
In general, the solubility of a slightly soluble salt is ________ by the presence of a second solute that furnishes a common ion.
(Short Answer)
4.9/5  (42)
(42)
Calculate the pH of a solution that is 0.322 M in nitrous acid (HNO2)and 0.178 M in potassium nitrite (KNO2). The acid dissociation constant of nitrous acid is 4.50 × 10-4.
(Multiple Choice)
4.8/5  (28)
(28)
Metal oxides and hydroxides that are relatively insoluble in neutral water, but are soluble in both strongly acidic and strongly basic solutions are said to be ________.
(Short Answer)
4.9/5  (33)
(33)
Determine the Ksp for magnesium hydroxide (Mg(OH)2)where the solubility of Mg(OH)2 is 1.4 × 10-4 M.
(Multiple Choice)
4.9/5  (31)
(31)
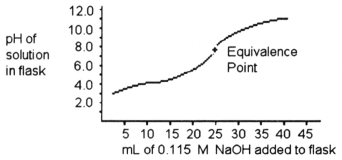 -A 25.0 mL sample of a solution of an unknown compound is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The unknown compound is ________.
-A 25.0 mL sample of a solution of an unknown compound is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. The unknown compound is ________.
(Multiple Choice)
4.9/5  (42)
(42)
A solution is prepared by dissolving 0.23 mol of benzoic acid and 0.27 mol of sodium benzoate in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly. The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution. The Ka of benzoic acid is 6.3 × 10-5.
(Multiple Choice)
4.8/5  (29)
(29)
What is the molar solubility of calcium carbonate ( CaCO3 )in water? The solubility-product constant for CaCO3 is 4.5 × 10-9 at 25 °C.
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the percent ionization of nitrous acid in a solution that is 0.241 M in nitrous acid (HNO2)and 0.195 M in potassium nitrite (KNO2). The acid dissociation constant of nitrous acid is 4.50 × 10-4.
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following will cause hemoglobin to release oxygen?
(Multiple Choice)
4.9/5  (40)
(40)
How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 0.0990 M HBr to the equivalence point?
(Multiple Choice)
4.7/5  (35)
(35)
Although CaCO3 has a relatively small solubility product, it is quite soluble in the presence of ________.
(Short Answer)
4.8/5  (34)
(34)
The Ka of benzoic acid is 6.30 × 10-5. The pH of a buffer prepared by combining 50.0 mL of 1.00 M potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
(Multiple Choice)
4.9/5  (39)
(39)
A buffer solution with a pH of 4.78 is prepared with ________ M formic acid and 0.90 M sodium formate. The Ka of formic acid is 1.8 × 10-4.
(Multiple Choice)
4.9/5  (40)
(40)
Why does fluoride treatment render teeth more resistant to decay?
(Multiple Choice)
4.7/5  (34)
(34)
The pH of a solution prepared by dissolving 0.350 mol of solid methylamine hydrochloride (CH3NH3Cl)in 1.00 L of 1.10 M methylamine (CH3NH2)is ________. The Kb for methylamine is 4.40 × 10-4. (Assume the final volume is 1.00 L.)
(Multiple Choice)
4.8/5  (41)
(41)
Showing 81 - 100 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)