Exam 12: Acids and Bases
Exam 1: The Quantum World99 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds84 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids94 Questions
Exam 7: Inorganic Materials99 Questions
Exam 8: Thermodynamics: the First Law94 Questions
Exam 9: Thermodynamics: the Second and Third Laws93 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria93 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics88 Questions
Exam 16: The Elements: the Main Group Elements186 Questions
Exam 17: The Elements: the D Block93 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I93 Questions
Exam 20: Organic Chemistry II94 Questions
Select questions type
In the following reaction
SO2(g)+ H2O(l) H2SO3(aq),
identify the Lewis acid and base.
(Short Answer)
4.7/5  (41)
(41)
The Ka of phenol is 1.3 1010.For a solution labeled "1.0 103 M aqueous phenol,"
(Multiple Choice)
4.8/5  (32)
(32)
In a solution labeled "0.10 M HNO3," which of the following is correct?
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following produces the strongest conjugate base?
(Multiple Choice)
4.9/5  (37)
(37)
For a solution of phosphoric acid,write the equation for (HPO42).
(Multiple Choice)
4.7/5  (29)
(29)
True or false: the pH of 0.10 M and 0.40 M NaHCO3(aq)solutions is 8.31 for both?
(True/False)
4.8/5  (29)
(29)
What is the pKa of the conjugate acid of hydrazine,given that the pKb of hydrazine is 5.77? Write the formula of the conjugate acid of hydrazine.
(Short Answer)
4.8/5  (44)
(44)
The fractional composition diagram for the amino acid alanine is shown below. 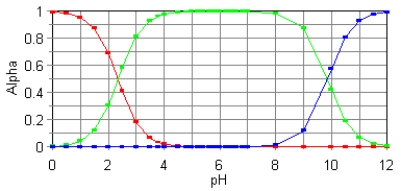 What do the two points represent where alpha is 0.5?
What do the two points represent where alpha is 0.5?
(Short Answer)
4.9/5  (41)
(41)
If (HSO3)= 0.83 at pH 2.5,what are (H2SO3)and (SO32)at this pH? For H2SO3,pKa1 and pKa2 are 1.81 and 6.91,respectively.
(Multiple Choice)
4.9/5  (28)
(28)
Write the charge balance equation for a dilute aqueous solution of KOH.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the hydrogen ion concentration for an aqueous solution that has a pH of 3.45.
(Multiple Choice)
4.8/5  (47)
(47)
The Ka of phenol is 1.3 1010.For a solution labeled "1.0 103 M aqueous phenol,"
(Multiple Choice)
4.7/5  (36)
(36)
Write the charge balance equation for a dilute aqueous solution of HI.
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)