Exam 1: Introduction to the Chemistry of Life
Exam 1: Introduction to the Chemistry of Life56 Questions
Exam 2: Water59 Questions
Exam 3: Nucleotides, Nucleic Acids, and Genetic Information68 Questions
Exam 4: Amino Acids68 Questions
Exam 5: Proteins: Primary Structure68 Questions
Exam 6: Proteins: Three-Dimensional Structure60 Questions
Exam 7: Protein Function Part I: Myoglobin and Hemoglobin74 Questions
Exam 8: Carbohydrates66 Questions
Exam 9: Lipids and Biological Membranes59 Questions
Exam 10: Membrane Transport50 Questions
Exam 11: Enzyme Catalysis53 Questions
Exam 12: Enzyme Kinetics, Inhibition and Control55 Questions
Exam 13: Biochemical Signaling50 Questions
Exam 14: Introduction to Metabolism52 Questions
Exam 15: Glycogen Metabolism and Gluconeogenesis50 Questions
Exam 16: Glucose Catabolism50 Questions
Exam 17: Citric Acid Cycle50 Questions
Exam 18: Electron Transport and Oxidative Phosphorylation50 Questions
Exam 19: Photosynthesis50 Questions
Exam 20: Lipid Metabolism51 Questions
Exam 21: Amino Acid Metabolism50 Questions
Exam 22: Mammalian Fuel Metabolism: Integration and Regulation50 Questions
Exam 23: Nucleotide Metabolism50 Questions
Exam 24: Nucleic Acid Structure50 Questions
Exam 25: Dna Replication, Repair and Recombination50 Questions
Exam 26: Transcription and Rna Processing50 Questions
Exam 27: Translation49 Questions
Exam 28: Regulation of Gene Expression50 Questions
Select questions type
Explain in one or two sentences why scientists believe that all living organisms are related (that they have all evolved from a common ancestor)?
Free
(Essay)
4.9/5  (30)
(30)
Correct Answer:
Biochemically, all living organisms are very similar.
Calculate the G for a reaction with H = 20.kJ/mol and S =20.J/K•mol, that is carried out at 27°C.
(Multiple Choice)
4.8/5  (40)
(40)
The term molecular weight is a term used by biochemists that refers to
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following developed during the evolution of eukaryotic cells from prokaryotic cells?
(Multiple Choice)
4.8/5  (47)
(47)
Matching
-The organisms most likely to be found in high temperature environments would be ______.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements concerning prokaryotes is false?
(Multiple Choice)
4.9/5  (38)
(38)
consider the structure of the coenzyme NADP 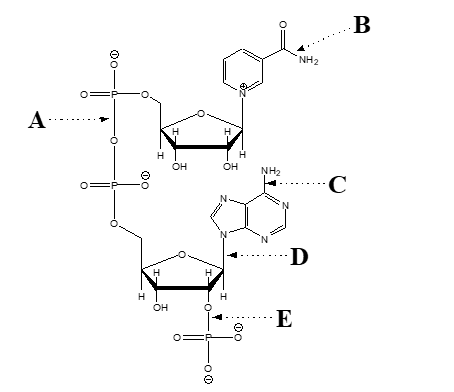 -Which arrow points at a phosphate ester bond?
-Which arrow points at a phosphate ester bond?
(Multiple Choice)
4.7/5  (45)
(45)
consider the structure of the coenzyme NADP  -Which arrow points at an amide bond?
-Which arrow points at an amide bond?
(Multiple Choice)
4.8/5  (39)
(39)
Phosphoglucomutase catalyses the reaction in which a phosphate group is transferred from the 1 carbon of glucose to the 6 carbon of glucose (G1P G6P).A student at SDSU incubates a 0.2 M solution of glucose-1-phosphate overnight with a small amount of the enzyme.At equilibrium the concentration of glucose 1-phosphate is 9.0 × 10−3 M and the concentration of glucose 6-phosphate is 19.1 × 10−2 M.
Calculate the equilibrium constant (Keq)and the standard state free energy (∆G°')for this reaction at 25°C.
(Essay)
4.8/5  (44)
(44)
Matching
-Living creatures can be described thermodynamically as ______ systems.
(Multiple Choice)
4.8/5  (45)
(45)
Consider a reaction in which H = -20.kJ/mol and S = 10.J/mol · K.
a.Calculate the G for this reaction at 25°C.
b.Is the reaction spontaneous (explain your answer)?
(Essay)
4.8/5  (36)
(36)
The bulk of aerobic metabolism in eukaryotic cells takes place in
(Multiple Choice)
4.7/5  (34)
(34)
Matching
-The symbol for free energy under standard biochemical conditions is ______.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)