Exam 5: The Atomic Nucleus
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
To predict the approximate energy release of either a fission or a fusion reaction, explain how a physicist uses a table of nuclear masses and the equation E = m 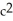 .
.
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
B
Why might the following nuclear reaction not be very good for energy production in a fission reactor? 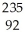 U →
U → 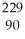 Th +
Th + 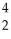 He + 3 neutrons
He + 3 neutrons
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
C
Which of the following statements best describes why a large nucleus is more likely to undergo radioactive decay?
Free
(Multiple Choice)
4.8/5  (30)
(30)
Correct Answer:
A
A sample of radium is usually a little warmer than its surroundings because ________.
(Multiple Choice)
4.9/5  (32)
(32)
Which type of radiation-alpha, beta, or gamma-results in the greatest change in atomic mass number?
(Multiple Choice)
4.8/5  (27)
(27)
Why is the carbon-14 dating not accurate for estimating the age of materials more than 50,000 years old?
(Multiple Choice)
5.0/5  (41)
(41)
The element uranium (U, atomic no. = 92) often has 146 (or more) neutrons but it will still undergo radioactive decay. Which statement might best describe why?
(Multiple Choice)
4.8/5  (30)
(30)
If an atom has 104 electrons, 157 neutrons, and 104 protons, what is its approximate atomic mass?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is a major disadvantage of nuclear-fission-based power plants?
(Multiple Choice)
4.8/5  (49)
(49)
A friend produces a Geiger counter to check the local background radiation. It ticks. Another friend, who normally fears most that which is understood least, makes an effort to keep away from the region of the Geiger counter and looks to you for advice. What do you say?
(Multiple Choice)
4.9/5  (40)
(40)
When 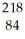 Po emits a beta particle, it transforms into a new element. What are the atomic number and atomic mass of this new element?
Po emits a beta particle, it transforms into a new element. What are the atomic number and atomic mass of this new element?
(Multiple Choice)
4.7/5  (38)
(38)
If a nucleus of 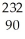 Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
Th absorbs a neutron and the resulting nucleus undergoes two successive beta decays (emitting electrons), what nucleus results?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following provides the minimum amount of protection you need to block the following form of radiation? alpha
(Multiple Choice)
4.7/5  (47)
(47)
The original reactor built in 1942 was just "barely" critical because the natural uranium that was used contained less than 1% of the fissionable isotope U-235 (half life 713 million years). What if, in 1942, the Earth had been 9 billion years old instead of 4.5 billion years old? Would this reactor have reached critical stage with natural uranium? Why?
(Multiple Choice)
4.9/5  (39)
(39)
The age of the Dead Sea Scrolls was found by carbon-14 dating. Could this technique have worked if they were carved in stone tablets? Explain.
(Multiple Choice)
4.8/5  (38)
(38)
Elements above uranium in the periodic table do not exist in any appreciable amounts in nature because they have short half-lives. Yet there are several elements below uranium in atomic number with equally short half-lives that do exist in appreciable amounts in nature. How can you account for this?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the beams is due to a positively charged helium nucleus?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)