Exam 5: The Atomic Nucleus
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Which are older, the atoms in the body of an elderly person or those in the body of a baby?
(Multiple Choice)
4.8/5  (46)
(46)
When  Ra decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? What is the resulting atomic mass?
Ra decays by emitting an alpha particle, what is the atomic number of the resulting nucleus? What is the resulting atomic mass?
(Multiple Choice)
4.9/5  (32)
(32)
Explain how radioactive decay has always warmed the earth from the inside, and nuclear fusion has always warmed the earth from the outside.
(Multiple Choice)
4.8/5  (34)
(34)
If carbon-14 is a beta emitter, what is the likely product of radioactive decay?
(Multiple Choice)
4.8/5  (43)
(43)
Why is the combination of two protons and two neutrons stable, but the combination of two protons and one neutron is not?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following statements about fusion is the most accurate?
(Multiple Choice)
4.7/5  (41)
(41)
Why might the following nuclear reaction not be very good for energy production in a fission reactor?  U →
U →  Ba +
Ba +  Kr + 1 neutron
Kr + 1 neutron
(Multiple Choice)
4.9/5  (39)
(39)
A pair of protons in a small atomic nucleus repel each other, but they are also attracted to each other. Why?
(Multiple Choice)
4.9/5  (42)
(42)
Why are smaller nuclei such as carbon-14 often radioactive? (carbon-12 is the most common and most stable form of carbon.)
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following nuclear equations correctly describes beta emission?
(Multiple Choice)
4.8/5  (33)
(33)
What does Einstein's energy equation (E = mc2) say about the energy that is derived from nuclear fission reactions?
(Multiple Choice)
4.9/5  (22)
(22)
Ordinary hydrogen is sometimes called a perfect fuel, because of its almost unlimited supply on Earth, and when it burns, harmless water is the product of the combustion. So why don't we abandon fission energy and fusion energy, not to mention fossil-fuel energy, and just use hydrogen?
(Multiple Choice)
4.8/5  (34)
(34)
Which type of radiation is being emitted in the following incomplete nuclear equation? 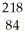 Po →
Po → 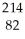 Pb + ??
Pb + ??
(Multiple Choice)
4.8/5  (35)
(35)
The half-life of carbon-14 is 5,730 years. A sample is found to have one-eighth the original amount of carbon-14 in it. How old is the sample?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements regarding a nucleon is true in regards to nuclear reactions?
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following statements best describes the strong nuclear force?
(Multiple Choice)
4.8/5  (31)
(31)
Which type of radiation is being emitted in the following incomplete nuclear equation? 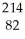 Pb →
Pb → 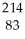 Bi + ??
Bi + ??
(Multiple Choice)
4.8/5  (29)
(29)
What is the main technical difficulty in dealing with fusion reactions?
(Multiple Choice)
4.7/5  (34)
(34)
If three samples have the half-lives listed below, which is the most radioactive?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)