Exam 4: Subatomic Particles
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
If two protons and two neutrons are removed from the nucleus of the oxygen-16 isotope, a nucleus of which element remains?
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
B
Which of these shows light waves in order of increasing energy?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
D
When we breathe we inhale oxygen, 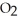 , and exhale carbon dioxide,
, and exhale carbon dioxide, 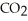 , plus water vapor,
, plus water vapor, 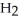 O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
O. Which likely has more mass, the air that we inhale or the same volume of air we exhale? Does breathing cause you to lose or gain weight?
(Multiple Choice)
4.9/5  (36)
(36)
How does Rutherford's model of the atom explain why some of the alpha particles directed at the gold foil were deflected straight back toward the source?
(Multiple Choice)
4.7/5  (45)
(45)
What is the main difference between a conceptual model and a physical model?
(Multiple Choice)
4.9/5  (35)
(35)
What property of an electron makes it possible to use electron microscopes?
(Multiple Choice)
4.8/5  (39)
(39)
Isotopes of the same element have a different number of ________.
(Multiple Choice)
4.9/5  (31)
(31)
The ________ represents the complete range of frequencies of light energy from radio waves to gamma rays.
(Multiple Choice)
4.8/5  (37)
(37)
Rank the following by the relative amounts of energy, from most energetic to least:
(Multiple Choice)
4.8/5  (36)
(36)
Does it make sense to say that a textbook is about 99.9 percent empty space?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements does not describe a proton?
(Multiple Choice)
4.8/5  (34)
(34)
If a neutral element has the following chemical notation, how many electrons does it have? fluorine-19
(Multiple Choice)
4.8/5  (39)
(39)
Which color of light comes from the higher energy transition, red or blue?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following best explains what is happening when an atom emits light?
(Multiple Choice)
4.9/5  (41)
(41)
What vibrates within an atom as it emits electromagnetic waves?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 1 - 20 of 146
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)