Exam 8: How Water Behaves
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds164 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
Consider a lake that is uniformly 10°C. What happens to the oxygen-rich surface water as it cools down to 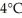 ?
?
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
Heat is given off by a substance when that substance ________.
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
B
Like water, hydrogen fluoride, HF, and ammonia, N 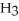 , have relatively high boiling points. Explain.
, have relatively high boiling points. Explain.
Free
(Multiple Choice)
5.0/5  (44)
(44)
Correct Answer:
C
What is the boiling temperature of a single water molecule? Does this question make sense?
(Multiple Choice)
4.9/5  (36)
(36)
From the diagram above describing the phase of water relative to temperature and pressure, at which point is water boiling?
(Multiple Choice)
4.8/5  (38)
(38)
If the specific heat of aluminum is 0.90 J/(g°C), how much heat is needed to raise the temperature of a 10.0-gram piece of aluminum from 21°C to 61°C?
(Multiple Choice)
4.9/5  (38)
(38)
Why is calcium chloride, 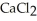 , more effective at melting ice than sodium chloride, NaCl?
, more effective at melting ice than sodium chloride, NaCl?
(Multiple Choice)
4.8/5  (36)
(36)
Why do ice cubes get smaller when left in the freezer for a long time?
(Multiple Choice)
4.8/5  (39)
(39)
What would happen to the oceans if the atmosphere suddenly disappeared?
(Multiple Choice)
4.7/5  (32)
(32)
Water coming out of volcanic sea vents at the bottom of the ocean can reach temperatures in excess of 300°C without boiling. How is this possible?
(Multiple Choice)
4.7/5  (33)
(33)
If it takes 200 J to raise the temperature of a 10-gram block of ice by 10°C, what is the specific heat of the ice?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the above liquids would most likely have the least capillary action?
(Multiple Choice)
4.8/5  (39)
(39)
Is the food compartment in a refrigerator cooled by evaporation or condensation of the refrigerating coolant?
(Multiple Choice)
4.7/5  (35)
(35)
Will wrapping a bottle in a wet cloth produce a cooler bottle than placing the bottle in a bucket of cold water?
(Multiple Choice)
4.9/5  (39)
(39)
What happens to the temperature of something while it is boiling?
(Multiple Choice)
4.7/5  (30)
(30)
Where is the boiling point of water the greatest: at the bottom of a pot of water or just below the surface?
(Multiple Choice)
4.9/5  (37)
(37)
Dip a paper clip into water and then slowly pull it out. You'll find that for a short distance, the water is brought up with the metal. Are these adhesive or cohesive forces at work?
(Multiple Choice)
4.9/5  (41)
(41)
Why does water expand when it goes from a liquid to a solid?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)