Exam 13: When Reactants Turn Into Products
Exam 1: What Is Chemistry51 Questions
Exam 2: The Numerical Side of Chemistry147 Questions
Exam 3: The Evolution of Atomic Theory92 Questions
Exam 4: The Modern Model of the Atom97 Questions
Exam 5: Chemical Bonding and Nomenclature245 Questions
Exam 6: The Shape of Molecules92 Questions
Exam 7: Intermolecular Forces and the Phases of Matter67 Questions
Exam 8: Chemical Reactions75 Questions
Exam 9: Stoichiometry and the Mole191 Questions
Exam 10: Electron Transfer in Chemical Reactions87 Questions
Exam 11: What If There Were No Intermolecular Forces the Ideal Gas80 Questions
Exam 12: Solutions92 Questions
Exam 13: When Reactants Turn Into Products79 Questions
Exam 14: Chemical Equilibrium94 Questions
Exam 15: Electrolytes, Acids, and Bases94 Questions
Exam 16: Nuclear Chemistry84 Questions
Exam 17: The Chemistry of Carbon71 Questions
Exam 18: Synthetic and Biological Polymers47 Questions
Select questions type
Consider the reaction A + B → Products. If the rate equation is Rate = -k[A]3[B]0 the order of the reaction with respect to A, B, and the total order respectively is ________.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following does not influence the speed of a chemical reaction?
(Multiple Choice)
4.9/5  (41)
(41)
 -In the diagram above, the two containers shown (Box A and BoxB)have exactly the same size (volume), are held at the same temperature, and contain reactant molecules (dots). In which box will the rate of reaction be faster?
-In the diagram above, the two containers shown (Box A and BoxB)have exactly the same size (volume), are held at the same temperature, and contain reactant molecules (dots). In which box will the rate of reaction be faster?
(Multiple Choice)
4.8/5  (28)
(28)
A reaction follows the rate law: Rate = -k[A]2[B]1. The overall order is ________.
(Multiple Choice)
4.7/5  (40)
(40)
A reaction follows the rate law: Rate = -k[A]3[B]-1. The overall order is ________.
(Multiple Choice)
4.7/5  (44)
(44)
Knowing the mechanism of a reaction is advantageous because one may influence its rate to make the reaction useful.
(True/False)
4.7/5  (35)
(35)
Which of the following is not true when the temperature of the reaction mixture is decreased?
(Multiple Choice)
4.8/5  (33)
(33)
Calculate a value for ΔErxn for a chemical reaction, if the reactants have an energy of +300 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?
(Short Answer)
4.9/5  (43)
(43)
The difference in energy between the reactants and products in a chemical reaction is called ________.
(Multiple Choice)
4.7/5  (39)
(39)
Calculate a value for ΔErxn for a chemical reaction if the reactants have an energy of -400 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?
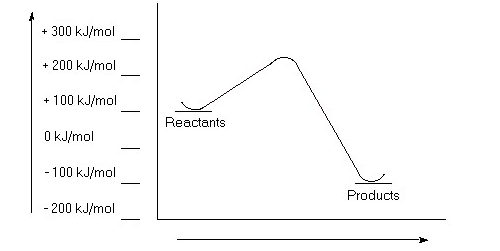 a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
(Short Answer)
4.8/5  (41)
(41)
In the reaction where Rate = [A]2, the rate is second order with respect to the reactant and second order overall.
(True/False)
4.8/5  (31)
(31)
The rate of reaction: A (g)+ 3B (g)→ C (g)+ 2D (g)is Rate = -k[A][B]3. If the concentration of B is doubled while that of A is unchanged, the rate will ________.
(Multiple Choice)
4.9/5  (36)
(36)
A catalyst lowers the activation energy for a chemical reaction.
(True/False)
4.7/5  (37)
(37)
It is possible to determine a given reaction's mechanism without performing any experiments of any kind.
(True/False)
4.8/5  (44)
(44)
Use the table below to answer the question that follows: ![Use the table below to answer the question that follows: -The above data were obtained in a kinetic study of the reaction. a)Determine the orders x and y, assuming that rate = k[NO]<sup>x</sup>[Cl<sub>2</sub>]<sup>y</sup>. b)Determine the value for the rate constant, k, in Experiment 2. c)Determine the overall order of this reaction.](https://storage.examlex.com/TB5644/11ea8554_738a_456f_9aec_054782e874fc_TB5644_00.jpg) -The above data were obtained in a kinetic study of the reaction.
a)Determine the orders x and y, assuming that rate = k[NO]x[Cl2]y.
b)Determine the value for the rate constant, k, in Experiment 2.
c)Determine the overall order of this reaction.
-The above data were obtained in a kinetic study of the reaction.
a)Determine the orders x and y, assuming that rate = k[NO]x[Cl2]y.
b)Determine the value for the rate constant, k, in Experiment 2.
c)Determine the overall order of this reaction.
(Essay)
4.8/5  (39)
(39)
For an endothermic reaction, the value for ΔErxn is a negative number.
(True/False)
4.7/5  (35)
(35)
The rate constant is dependant on the activation energy and reactant orientation.
(True/False)
4.9/5  (30)
(30)
Match the rate of each reaction in the questions with the overall order that appears in the list below.
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (41)
(41)
Showing 21 - 40 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)