Exam 13: When Reactants Turn Into Products
Exam 1: What Is Chemistry51 Questions
Exam 2: The Numerical Side of Chemistry147 Questions
Exam 3: The Evolution of Atomic Theory92 Questions
Exam 4: The Modern Model of the Atom97 Questions
Exam 5: Chemical Bonding and Nomenclature245 Questions
Exam 6: The Shape of Molecules92 Questions
Exam 7: Intermolecular Forces and the Phases of Matter67 Questions
Exam 8: Chemical Reactions75 Questions
Exam 9: Stoichiometry and the Mole191 Questions
Exam 10: Electron Transfer in Chemical Reactions87 Questions
Exam 11: What If There Were No Intermolecular Forces the Ideal Gas80 Questions
Exam 12: Solutions92 Questions
Exam 13: When Reactants Turn Into Products79 Questions
Exam 14: Chemical Equilibrium94 Questions
Exam 15: Electrolytes, Acids, and Bases94 Questions
Exam 16: Nuclear Chemistry84 Questions
Exam 17: The Chemistry of Carbon71 Questions
Exam 18: Synthetic and Biological Polymers47 Questions
Select questions type
Which of the following can be done to increase the likelihood that a chemical reaction will take place?
(Multiple Choice)
4.9/5  (34)
(34)
The reaction energy profile can tell if a reaction is exothermic or endothermic.
(True/False)
4.8/5  (27)
(27)
Which of the following is an example of a substitution reaction?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following "adjustments" can be made to a chemical reaction system to increase the rate of reaction?
(Multiple Choice)
4.7/5  (35)
(35)
In the following equation 2Y (g)→ Z (g)at 20 °C the following data were obtained:  The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
4.9/5  (43)
(43)
The rate constant of a reaction may be increased by ________.
(Multiple Choice)
4.7/5  (32)
(32)
If the products have a higher energy than the reactants ________.
(Multiple Choice)
4.8/5  (35)
(35)
Reaction rates can be varied by changing either the concentrations of the reacting species or the temperature at which the reaction is carried out.
(True/False)
4.9/5  (37)
(37)
In each of the following diagrams, the x-axis is the reaction coordinate and the y-axis is the potential energy. Which diagram corresponds to a reaction that has an activation energy of 35 kJ and an overall reaction energy of -110 kJ?
(Multiple Choice)
4.7/5  (37)
(37)
In the following equation 2 Y (g)+ X (g)→ Z (g)at 100 °C the following data were obtained: 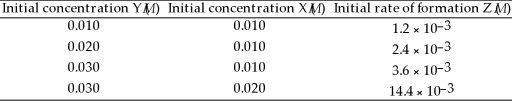 The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
5.0/5  (33)
(33)
Consider the reaction X → Y with the following data: Energy of reactants = 50 kJ/mole;
Energy of reaction = -30 kJ/mole;
Energy of transition state = 90 kJ/mole.
The energy of products for the reaction is ________ kJ/mole.
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 79 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)