Exam 13: When Reactants Turn Into Products
Exam 1: What Is Chemistry51 Questions
Exam 2: The Numerical Side of Chemistry147 Questions
Exam 3: The Evolution of Atomic Theory92 Questions
Exam 4: The Modern Model of the Atom97 Questions
Exam 5: Chemical Bonding and Nomenclature245 Questions
Exam 6: The Shape of Molecules92 Questions
Exam 7: Intermolecular Forces and the Phases of Matter67 Questions
Exam 8: Chemical Reactions75 Questions
Exam 9: Stoichiometry and the Mole191 Questions
Exam 10: Electron Transfer in Chemical Reactions87 Questions
Exam 11: What If There Were No Intermolecular Forces the Ideal Gas80 Questions
Exam 12: Solutions92 Questions
Exam 13: When Reactants Turn Into Products79 Questions
Exam 14: Chemical Equilibrium94 Questions
Exam 15: Electrolytes, Acids, and Bases94 Questions
Exam 16: Nuclear Chemistry84 Questions
Exam 17: The Chemistry of Carbon71 Questions
Exam 18: Synthetic and Biological Polymers47 Questions
Select questions type
In the following equation 2W (g)→ V (g)at 20 °C the following data were obtained: 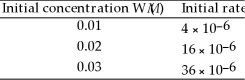 The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
4.9/5  (36)
(36)
The reactants must collide with an energy ≥ the energy of activation for the reaction to occur.
(True/False)
4.8/5  (40)
(40)
Which of the following is not a term involved in determining the overall rate of a chemical reaction?
(Multiple Choice)
4.8/5  (39)
(39)
Generally in a biochemical reaction the first step involves ________.
(Multiple Choice)
4.7/5  (38)
(38)
The slow step in the multistep mechanism of a reaction ________.
(Multiple Choice)
4.8/5  (46)
(46)
Adding a catalyst increases the rate of reaction by providing the additional energy needed to overcome a large energy of activation.
(True/False)
4.9/5  (34)
(34)
A lower energy of activation leads to more products favored in the reaction.
(True/False)
4.8/5  (33)
(33)
For an exothermic reaction, the value for ΔErxn is a negative number.
(True/False)
4.8/5  (30)
(30)
Based on the collision theory, adding a catalyst to a reaction increases the reaction rate.
(True/False)
4.9/5  (49)
(49)
Based on the collision theory, decreasing temperature for a reaction increases the reaction rate.
(True/False)
4.8/5  (35)
(35)
Which of the following responses contains the two rate factors that comprise the rate constant (k)for a given chemical reaction?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements regarding reaction mechanisms is true?
(Multiple Choice)
4.8/5  (34)
(34)
Examine this reaction coordinate diagram and answer the questions that follow. 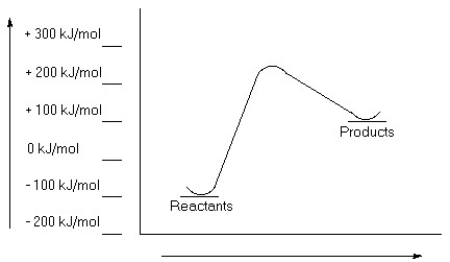 a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
(Short Answer)
4.8/5  (38)
(38)
The fraction of collisions with the proper orientation depends on ________.
(Multiple Choice)
4.8/5  (39)
(39)
The reaction A + B + C → Products follows the rate law: Rate = - k[A]1[B]-1[C]2. The overall rate of this reaction is ________.
(Multiple Choice)
4.9/5  (32)
(32)
If the forward reaction is exothermic, the reverse is endothermic.
(True/False)
4.8/5  (34)
(34)
When the heat of reaction is a positive number, the reaction is exothermic.
(True/False)
4.8/5  (46)
(46)
The greater the concentration of the reactants, the greater the rate of the reaction.
(True/False)
4.9/5  (40)
(40)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)