Exam 6: Liquids and Solids
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
The compound KCN·2H2O is likely to be commonly available and thus found in the laboratory.True or false?
(True/False)
4.7/5  (49)
(49)
Which of the following has the highest molar enthalpy of vaporization?
(Multiple Choice)
4.9/5  (37)
(37)
If the interaction between two species is proportional to 1/r6,which of the following is likely involved?
(Multiple Choice)
4.9/5  (45)
(45)
How many calcium and fluorine ions are there in the fluorite unit cell shown below? 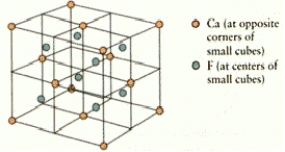
(Multiple Choice)
4.7/5  (46)
(46)
Gold has a face-centered cubic unit cell.If the edge length of the unit cell is 407 pm,what is the atomic radius of gold?
(Short Answer)
4.8/5  (41)
(41)
Water was found to rise to a height of 7.4 mm in a tube of internal radius 2.0 mm at room temperature.Given its density as 1.0 g.cm-3,what is the surface tension of water?
(Multiple Choice)
4.8/5  (39)
(39)
What are all the intermolecular forces that are responsible for the existence of the molecular solid oxalic acid,H2C2O4?
(Multiple Choice)
4.7/5  (37)
(37)
In the rock-salt structure (NaCl),the cations occupy all the octahedral holes.True or false?
(True/False)
4.9/5  (36)
(36)
Which of the following has the largest value of surface tension?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following can form intermolecular hydrogen bonds?
(Multiple Choice)
4.9/5  (45)
(45)
Which answer best accounts for the Cl- ions present in the NaCl unit cell?
(Multiple Choice)
4.8/5  (45)
(45)
All the following hydrated compounds are commonly available except
(Multiple Choice)
4.9/5  (41)
(41)
What is the coordination number of cesium in CsCl? The ionic radii of Cs+ and Cl- are 170 and 181 pm,respectively.
(Multiple Choice)
5.0/5  (36)
(36)
Which has the higher boiling point,1,1-dichloroethene or cis-dichloroethene?
(Short Answer)
4.8/5  (34)
(34)
Showing 41 - 60 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)