Exam 6: Liquids and Solids
Exam 1: The Quantum World100 Questions
Exam 2: Quantum Mechanics in Action: Atoms100 Questions
Exam 3: Chemical Bonds83 Questions
Exam 4: Molecular Shape and Structure95 Questions
Exam 5: The Properties of Gases94 Questions
Exam 6: Liquids and Solids95 Questions
Exam 7: Inorganic Materials100 Questions
Exam 8: Thermodynamics: The First Law94 Questions
Exam 9: Thermodynamics: The Second and Third Laws95 Questions
Exam 10: Physical Equilibria94 Questions
Exam 11: Chemical Equilibria94 Questions
Exam 12: Acids and Bases94 Questions
Exam 13: Aqueous Equilibria94 Questions
Exam 14: Electrochemistry94 Questions
Exam 15: Chemical Kinetics93 Questions
Exam 16: The Elements: the Main Group Elements189 Questions
Exam 17: The Elements: The D Block94 Questions
Exam 18: Nuclear Chemistry95 Questions
Exam 19: Organic Chemistry I94 Questions
Exam 20: Organic Chemistry II95 Questions
Select questions type
Which of the following cations is likely to be hydrated in compounds?
(Multiple Choice)
4.8/5  (41)
(41)
Which answer best accounts for the Na+ ions present in the NaCl unit cell?
(Multiple Choice)
4.8/5  (33)
(33)
If the radius of an atom is r,what is the length of the side of the body-centered cubic unit cell?
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following can form intermolecular hydrogen bonds?
(Multiple Choice)
4.8/5  (38)
(38)
What are the coordination numbers of Ca2+ and F- ions,respectively,in fluorite? The unit cell is shown below 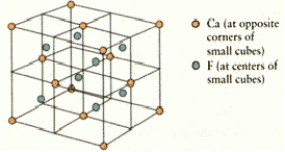
(Multiple Choice)
4.8/5  (36)
(36)
Tetrabromomethane has a higher boiling point than tetrachloromethane.True or false?
(True/False)
5.0/5  (40)
(40)
Which of the following can form intermolecular hydrogen bonds?
(Multiple Choice)
4.8/5  (38)
(38)
What is the formula of the superconductor whose unit cell is shown below? (The Y and Ba atoms are in the middle of the cell.) 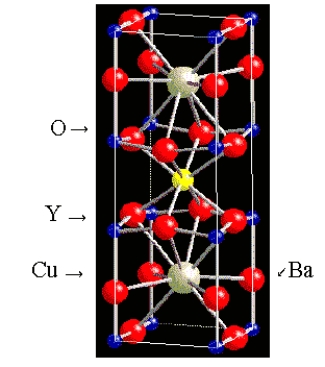
(Multiple Choice)
4.8/5  (32)
(32)
If the radius of an atom is r,what is the length of the side of the face-centered cubic unit cell?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following cations is likely to be hydrated in compounds?
(Multiple Choice)
5.0/5  (45)
(45)
How many octahedral holes are there in a face-centered cubic unit cell?
(Multiple Choice)
4.9/5  (32)
(32)
An amorphous solid is one in which the atoms,ions,or molecules lie in a random jumble with no order.True or false?
(True/False)
4.8/5  (30)
(30)
A metal with a cubic close-packed structure is malleable because
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is the strongest intermolecular force between molecules?
(Multiple Choice)
4.8/5  (35)
(35)
What are the coordination numbers of Ti4+ and O2-,respectively,in rutile? The unit cell is shown below. 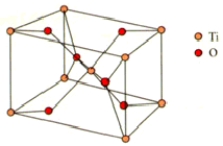
(Multiple Choice)
4.9/5  (37)
(37)
How many calcium,titanium,and oxygen ions are there in the perovskite unit cell shown below? 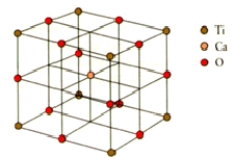
(Multiple Choice)
4.8/5  (38)
(38)
Estimate the density of cesium iodide from its crystal structure.The ionic radii of Cs+ and Cl- are 170 and 220 pm,respectively.
(Multiple Choice)
4.8/5  (44)
(44)
Showing 61 - 80 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)