Exam 11: Liquids,solids,and Intermolecular Forces
Exam 1: Matter, measurement, and Problem Solving170 Questions
Exam 2: Atoms and Elements157 Questions
Exam 3: Molecules,compounds,and Chemical Equations175 Questions
Exam 4: Chemical Quantities and Aqueous Reactions239 Questions
Exam 5: Gases182 Questions
Exam 6: Thermochemistry143 Questions
Exam 7: The Quantum-Mechanical Model of the Atom134 Questions
Exam 8: Periodic Properties of the Elements147 Questions
Exam 9: Chemical Bonding I: Lewis Theory166 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,144 Questions
Exam 11: Liquids,solids,and Intermolecular Forces128 Questions
Exam 12: Solids and Modern Materials81 Questions
Exam 13: Solutions157 Questions
Exam 14: Chemical Kinetics154 Questions
Exam 15: Chemical Equilibrium141 Questions
Exam 16: Acids and Bases160 Questions
Exam 17: Aqueous Ionic Equilibrium187 Questions
Exam 18: Free Energy and Thermodynamics130 Questions
Exam 19: Electrochemistry151 Questions
Exam 20: Radioactivity and Nuclear Chemistry135 Questions
Exam 21: Organic Chemistry104 Questions
Exam 22: Biochemistry68 Questions
Exam 23: Chemistry of the Nonmetals66 Questions
Exam 24: Metals and Metallurgy60 Questions
Exam 25: Transition Metals and Coordination Compounds73 Questions
Select questions type
Place the following substances in order of decreasing boiling point. F2 Br2 H2
(Multiple Choice)
4.7/5  (44)
(44)
Place the following compounds in order of increasing boiling point. LiF N2 CH3CH2NH2
(Multiple Choice)
4.8/5  (38)
(38)
The enthalpy change for converting 10.0 g of ice at -50.0°C to water at 70.0°C is ________ kJ.The specific heats of ice,water,and steam are 2.09 J/gK,4.18 J/gK,and
1)84 J/gK,respectively.For H2O,ΔHfus = 6.01 kJ/mol,and ΔHvap = 40.67 kJ/mol.
(Multiple Choice)
4.9/5  (41)
(41)
Place the following compounds in order of decreasing strength of intermolecular forces. HF F2 CO2
(Multiple Choice)
4.9/5  (33)
(33)
For automobile engines,identify the best choice of motor oil.
(Multiple Choice)
4.9/5  (32)
(32)
The fluorocarbon C2Cl3F3 has a normal boiling point of 47.6°C.The specific heats of C2Cl3F3(l)and C2Cl3F3(g)are 0.91 J/gK and 0.67 J/gK,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 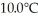 to the gas at 65.0°C is ________ kJ.
to the gas at 65.0°C is ________ kJ.
(Multiple Choice)
4.8/5  (34)
(34)
The heat of vaporization of water at 100°C is 40.66 kJ/mol.Calculate the quantity of heat that is absorbed/released when 6.00 g of steam condenses to liquid water at 100°C.
(Multiple Choice)
4.8/5  (55)
(55)
Place the following substances in order of decreasing vapor pressure at a given temperature. PF5 BrF3 CF4
(Multiple Choice)
4.9/5  (31)
(31)
Determine the vapor pressure (in torr)of a substance at 36°C,whose normal boiling point is 84°C and has a ΔHvap of 22.1 kJ/mol.
(Multiple Choice)
4.8/5  (39)
(39)
Identify the state of matter that is the most compressible.
(Multiple Choice)
4.9/5  (42)
(42)
Showing 81 - 100 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)