Exam 1: Introduction to the Chemistry of Life
Exam 1: Introduction to the Chemistry of Life56 Questions
Exam 2: Water59 Questions
Exam 3: Nucleotides, nucleic Acids, and Genetic Information67 Questions
Exam 4: Amino Acids68 Questions
Exam 5: Proteins: Primary Structure68 Questions
Exam 6: Proteins: Three-Dimensional Structure60 Questions
Exam 7: Protein Function Part I: Myoglobin and Hemoglobin74 Questions
Exam 8: Carbohydrates66 Questions
Exam 9: Lipids and Biological Membranes58 Questions
Exam 10: Membrane Transport49 Questions
Exam 11: Enzyme Catalysis53 Questions
Exam 12: Enzyme Kinetics, inhibition and Control54 Questions
Exam 13: Biochemical Signaling50 Questions
Exam 14: Introduction to Metabolism52 Questions
Exam 15: Glucose Catabolism50 Questions
Exam 16: Glycogen Metabolism and Gluconeogenesis50 Questions
Exam 17: Citric Acid Cycle50 Questions
Exam 18: Electron Transport and Oxidative Phosphorylation50 Questions
Exam 19: Photosynthesis50 Questions
Exam 20: Lipid Metabolism51 Questions
Exam 21: Amino Acid Metabolism50 Questions
Exam 22: Mammalian Fuel Metabolism: Integration and Regulation50 Questions
Exam 23: Nucleotide Metabolism50 Questions
Exam 24: Nucleic Acid Structure50 Questions
Exam 25: DNA Replication, repair and Recombination50 Questions
Exam 26: Transcription and Rna Processing50 Questions
Exam 27: Protein Synthesis49 Questions
Exam 28: Regulation of Gene Expression50 Questions
Select questions type
Matching
-The symbol for free energy under standard biochemical conditions is ______.
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
K
Which cellular compartment or organelle is involved in the synthesis of proteins and lipids?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
A
Which three cellular components are present in both prokaryotes and eukaryotes?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
C
For the followin questions,consider the structure of the coenzyme NADP 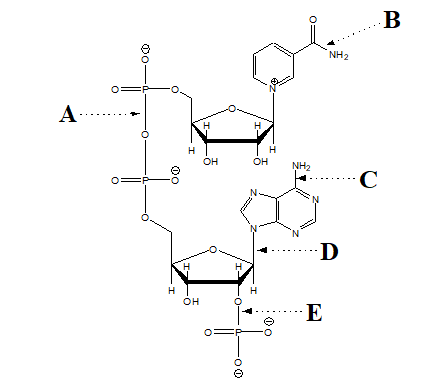 -Which arrow points at a phosphate ester bond?
-Which arrow points at a phosphate ester bond?
(Multiple Choice)
4.9/5  (36)
(36)
Carbohydrates,proteins,nucleotides,and nucleic acids are important groups of molecules found in living organisms.Which 6 elements do living organisms need to build these molecules?
(Short Answer)
4.7/5  (28)
(28)
The reaction pyruvate + ATP PEP + ADP is catalyzed by the enzyme pyruvate kinase.The G°ꞌ for this reaction is 31.4 kJ/mol.Use this information to answer the following questions.
a.Does this reaction move forward (from left to right)or backward under standard state conditions? Explain you answer in one sentence.
b.Calculate the Keq for this reaction at 25°C.
c.After the reaction has reached equilibrium at 25°C [ATP] = 5 mM,[ADP] = 0.5 mM,[pyruvate] = 0.2 mM.Calculate the concentration PEP.
(Essay)
4.9/5  (40)
(40)
Consider the reaction A + B C + D.After reaching equilibrium at 25°C,the following concentrations of reactants and products were measured: [A] = 10 M,[B] = 15 M,[C] = 10 M,[D] = 10 M.Calculate G°' for this reaction.
(Multiple Choice)
4.9/5  (37)
(37)
For a reaction with H = 23 kJ/mol and S =22 J/K•mol,at 2°C,the reaction is:
(Multiple Choice)
4.8/5  (39)
(39)
For the followin questions,consider the structure of the coenzyme NADP  -Which arrow points at a glycosidic bond?
-Which arrow points at a glycosidic bond?
(Multiple Choice)
4.8/5  (26)
(26)
Calculate the G for a reaction with H = 20.kJ/mol and S =20.J/K•mol,that is carried out at 27°C.
(Multiple Choice)
4.7/5  (35)
(35)
For the followin questions,consider the structure of the coenzyme NADP  -Which arrow points at an amide bond?
-Which arrow points at an amide bond?
(Multiple Choice)
4.8/5  (27)
(27)
Matching
-The term used to indicate the degree of randomness within a system is ______.
(Multiple Choice)
4.8/5  (40)
(40)
For the followin questions,consider the structure of the coenzyme NADP  -Which arrow points at an anhydride bond?
-Which arrow points at an anhydride bond?
(Multiple Choice)
4.9/5  (34)
(34)
Matching
-Spontaneous processes are characterized by a change in Gibbs free energy that is ______.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements about hydrothermal vents is true?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)