Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion30 Questions
Exam 2: Motion in One Dimension54 Questions
Exam 3: Vectors and Motion in Two Dimensions57 Questions
Exam 4: Forces and Newtons Laws of Motion60 Questions
Exam 5: Applying Newtons Laws59 Questions
Exam 6: Circular Motion,orbits,and Gravity60 Questions
Exam 7: Rotational Motion37 Questions
Exam 8: Equilibrium and Elasticity39 Questions
Exam 9: Momentum43 Questions
Exam 10: Energy and Work50 Questions
Exam 11: Using Energy36 Questions
Exam 12: Thermal Properties of Matter79 Questions
Exam 13: Fluids51 Questions
Exam 14: Oscillations41 Questions
Exam 15: Traveling Waves and Sound45 Questions
Exam 16: Superposition and Standing Waves45 Questions
Exam 17: Wave Optics51 Questions
Exam 18: Ray Optics58 Questions
Exam 19: Optical Instruments34 Questions
Exam 20: Electric Fields and Forces60 Questions
Exam 21: Electric Potential46 Questions
Exam 22: Current and Resistance33 Questions
Exam 23: Circuits44 Questions
Exam 24: Magnetic Fields and Forces47 Questions
Exam 25: Electromagnetic Induction and Electromagnetic Waves66 Questions
Exam 26: Ac Electricity35 Questions
Exam 27: Relativity48 Questions
Exam 28: Quantum Physics66 Questions
Exam 29: Atoms and Molecules48 Questions
Exam 30: Nuclear Physics48 Questions
Select questions type
Which contains more moles of material: 80 grams of helium or 400 grams of argon?
(Multiple Choice)
4.9/5  (38)
(38)
A 920 g empty iron kettle is put on a stove.How much heat in joules must it absorb to raise its temperature form 15.0ºC to 93.0ºC? (The specific heat for iron is 113 cal/kg · ºC. )
(Multiple Choice)
4.8/5  (29)
(29)
At what temperature would the average thermal speed of oxygen molecules be 13.0 m/s? Oxygen is assumed to approximate an ideal gas.The mass of one O2 molecule is 5.312 × 10-26 kg.
(Multiple Choice)
4.7/5  (29)
(29)
In years of heavy snow pack in the mountains it is sometimes desirable to induce early melting of the snow,rather than wait until it all melts suddenly and causes floods.It has been suggested that a way to accomplish this might be to have planes fly over the snow fields and sprinkle them with black soot.What do you think of this idea? Would it work?
(Multiple Choice)
4.8/5  (36)
(36)
A heat conducting rod,0.90 m long,is made of an aluminum section,0.20 m long,and a copper section,0.70 m long.Both sections have a cross-sectional area of 0.0004 m2.The aluminum end and the copper end are maintained at temperatures of 30ºC and 230ºC,respectively.The thermal conductivities of aluminum and copper are 205 and 385 W/m ∙ K,respectively.The temperature of the aluminum-copper junction in the rod,in ºC,is closest to:
(Multiple Choice)
4.9/5  (33)
(33)
A constant-volume gas thermometer is filled with air whose pressure is 104 Pa at the triple point of water.What would the pressure be at 32.0 K?
(Multiple Choice)
4.9/5  (35)
(35)
A sealed 29 m3 tank is filled with 9000 moles of ideal oxygen gas (diatomic)at an initial temperature of 270 K.The gas is heated to a final temperature of 330 K.The atomic mass of oxygen is 16.0 g/mol.The initial pressure of the gas,in MPa,is closest to:
(Multiple Choice)
4.9/5  (30)
(30)
How many moles of an ideal gas are there in a container with a pressure of 108,893 Pa,a temperature of 321 K,and a volume of 2.0 L? The universal gas constant is 8.314 J/K · mol.
(Multiple Choice)
4.9/5  (39)
(39)
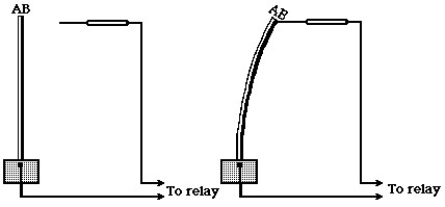 Shown here is a device that can be used to turn a furnace on or off, depending on the temperature sensed by the device.
-In the figure,the principle underlying the operation of this device is that
Shown here is a device that can be used to turn a furnace on or off, depending on the temperature sensed by the device.
-In the figure,the principle underlying the operation of this device is that
(Multiple Choice)
4.7/5  (43)
(43)
The figure shows 0.0074 mol of gas that undergoes the process 1 → 2 → 3.What is the volume V3? 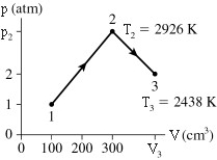
(Multiple Choice)
4.8/5  (35)
(35)
As 771.0 kg copper bar is put into a smelter for melting.The initial temperature of the copper is 300.0 K.How much heat must the smelter produce to completely melt the copper bar? (The specific heat for copper is 386 J/kg · K,the heat of fusion for copper is 205 kJ/kg,and its melting point is 1357 K. )
(Multiple Choice)
4.8/5  (40)
(40)
If two different gases are at the same temperature,the root mean square speed of their molecules must be the same for both of them.
(True/False)
4.8/5  (39)
(39)
A person consumes a large meal containing 14 kcal.What is the power this meal produces if it is to be "burned off" due to exercise in 6 hours?
(Multiple Choice)
4.8/5  (45)
(45)
An ideal gas is held in a container of volume V at pressure P.The rms speed of a gas molecule under these conditions is v.If now the volume and pressure are changed to 2V and 2P,the rms speed of a molecule will be:
(Multiple Choice)
4.9/5  (32)
(32)
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of heat was added to it,which of the following would be most helpful to know?
(Multiple Choice)
4.9/5  (24)
(24)
Some properties of glass are listed here.  A glass window pane is 2.7 m high,2.4 m wide and 9 mm thick.The temperature at the inner surface of the glass is 19ºC and at the outer surface 4°C.How much heat is lost each hour through the window?
A glass window pane is 2.7 m high,2.4 m wide and 9 mm thick.The temperature at the inner surface of the glass is 19ºC and at the outer surface 4°C.How much heat is lost each hour through the window?
(Multiple Choice)
4.8/5  (23)
(23)
In an isothermal process,the gas temperature always remains constant and the PV diagram is a hyperbola.
(True/False)
4.7/5  (36)
(36)
1.0 mol of an elemental solid and 1.0 mol of a monatomic gas interact thermally.What is the temperature change of the solid if the gas temperature decreases by 60°C at constant volume?
(Multiple Choice)
4.8/5  (33)
(33)
A 960.0 g iron meteor impacts the earth at a speed of 1268 m/s.If its kinetic energy is entirely converted to heat of the meteorite,what will the resultant temperature rise be? (The specific heat for iron is 113 cal/kg · °C. )
(Multiple Choice)
4.9/5  (40)
(40)
The diagrams shows a PV diagram for 4.3 g of oxygen gas in a sealed container.The temperature of state 1 is 21°C.What are the temperatures T3 and T4? 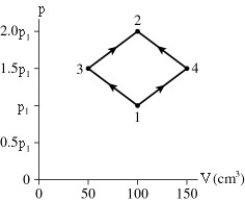
(Multiple Choice)
4.7/5  (34)
(34)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)