Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion30 Questions
Exam 2: Motion in One Dimension54 Questions
Exam 3: Vectors and Motion in Two Dimensions57 Questions
Exam 4: Forces and Newtons Laws of Motion60 Questions
Exam 5: Applying Newtons Laws59 Questions
Exam 6: Circular Motion,orbits,and Gravity60 Questions
Exam 7: Rotational Motion37 Questions
Exam 8: Equilibrium and Elasticity39 Questions
Exam 9: Momentum43 Questions
Exam 10: Energy and Work50 Questions
Exam 11: Using Energy36 Questions
Exam 12: Thermal Properties of Matter79 Questions
Exam 13: Fluids51 Questions
Exam 14: Oscillations41 Questions
Exam 15: Traveling Waves and Sound45 Questions
Exam 16: Superposition and Standing Waves45 Questions
Exam 17: Wave Optics51 Questions
Exam 18: Ray Optics58 Questions
Exam 19: Optical Instruments34 Questions
Exam 20: Electric Fields and Forces60 Questions
Exam 21: Electric Potential46 Questions
Exam 22: Current and Resistance33 Questions
Exam 23: Circuits44 Questions
Exam 24: Magnetic Fields and Forces47 Questions
Exam 25: Electromagnetic Induction and Electromagnetic Waves66 Questions
Exam 26: Ac Electricity35 Questions
Exam 27: Relativity48 Questions
Exam 28: Quantum Physics66 Questions
Exam 29: Atoms and Molecules48 Questions
Exam 30: Nuclear Physics48 Questions
Select questions type
The amount of heat needed to raise the temperature of one gram of an ideal gas by 1.0 K at constant volume is the same for all monatomic gases.
(True/False)
4.9/5  (40)
(40)
The amount of heat needed to raise the temperature of one mole of an ideal gas by 1.0 K at constant volume is the same for all monatomic gases.
(True/False)
4.7/5  (30)
(30)
Dust particles are pulverized rock,which has density 2500kg/m3.They are approximately spheres with 10μm in diameter.Treating dust as an ideal gas,what is the rms speed of a dust particle at 400°C?
(Multiple Choice)
4.7/5  (27)
(27)
The figure shows a PV diagram for 8.3 g of nitrogen gas in a sealed container.The temperature of state 1 is 79°C.What are (a)pressure p1 and (b)temperature T2? 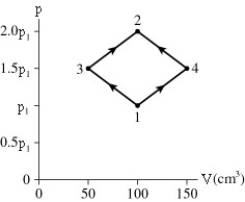
(Multiple Choice)
4.8/5  (32)
(32)
A 24.0 kg sample of ice is at 0.00°C.How much heat is needed to melt it? (For water Lf = 334 kJ/kg and Lv = 2257 kJ/kg. )
(Multiple Choice)
5.0/5  (40)
(40)
A cylinder fitted with a movable piston contains ideal gas at 27°C,pressure 0.50 x 105 Pa and volume 1.25 m3.What will be the final temperature if the gas is compressed to 0.80 m3 and the pressure rises to 0.82 × 105 Pa?
(Multiple Choice)
4.7/5  (37)
(37)
The temperature is increased from 20°C to 180°C.By what factor does the rms speed of a molecule change?
(Multiple Choice)
4.8/5  (44)
(44)
As you add heat to boiling water,its temperature gets higher and higher.
(True/False)
4.9/5  (43)
(43)
The oxygen molecules and the nitrogen molecules at any one place in our atmosphere are at the same temperature.How do the root-mean-square speeds of the two molecules compare?
(Multiple Choice)
4.8/5  (31)
(31)
A horizontal line on a PV diagram represents an isobaric process.
(True/False)
4.8/5  (35)
(35)
A hot air balloon has a volume of 2000 m3 when fully inflated,and the air inside the balloon is always at atmospheric pressure because of the large opening used to fill the balloon and heat the air inside it.What's the mass of hot air inside the balloon if its temperature is 120°C? (Assume a molecular weight of 28.8 g/mole for air. )
(Multiple Choice)
4.8/5  (33)
(33)
An ideal gas is in a closed container.If its pressure is 133 Pa initially,and its temperature is 20.0ºC,what is its pressure after its temperature is raised to 60.0ºC?
(Multiple Choice)
4.9/5  (34)
(34)
A 2294 kg sample of water at 0°C is cooled to -36ºC,and freezes in the process.How much heat is liberated? (For water Lf = 334 kJ/kg and Lv = 2257 kJ/kg.The specific heat for ice is 2050 J/kg · K. )
(Multiple Choice)
4.9/5  (34)
(34)
A constant-volume gas thermometer supports a 33 mm high column of mercury when its temperature is 216 K.What will the height of the column of mercury be when the temperature is 298 K? Answer to the nearest millimeter.
(Multiple Choice)
4.8/5  (26)
(26)
If the root mean square speed of gas molecules is doubled,then both its pressure and its absolute temperature will also be doubled.
(True/False)
4.7/5  (31)
(31)
A gas follows the pV trajectory shown in the figure.How much work is done per cycle by the gas if p0 = 5.4 atm? 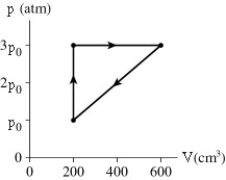
(Multiple Choice)
4.9/5  (38)
(38)
A monatomic ideal gas (Cv = 3/2 R)undergoes an isothermal expansion at 300 K,as the volume increased from 0.05 m3 to 0.15 m3.The final pressure is 130 kPa.The heat transfer to the gas,in kJ,is closest to:
(Multiple Choice)
4.7/5  (49)
(49)
If a piece of metal has a hole in it and the metal is heated,how does the area of the hole change?
(Short Answer)
4.9/5  (29)
(29)
Showing 61 - 79 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)