Exam 3: An Introduction to Organic Reactions: Acids and Bases
Exam 1: Carbon Compounds and Chemical Bonds134 Questions
Exam 2: Representative Carbon Compounds: Functional Groups, Intermolecular Forces, and Infrared Ir Spectroscopy114 Questions
Exam 3: An Introduction to Organic Reactions: Acids and Bases47 Questions
Exam 4: Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis125 Questions
Exam 5: Stereochemistry: Chiral Molecules150 Questions
Exam 6: Ionic Reactions - Nucleophilic Substitution and Elimination Reactions of Alkyl Halides146 Questions
Exam 7: Alkenes and Alkynes I: Properties and Synthesis, Elimination Reactions of Alkyl Halides99 Questions
Exam 8: Alkenes and Alkynes Ii: Addition Reactions140 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry: Tools for Structure Determination94 Questions
Exam 10: Radical Reactions114 Questions
Exam 11: Alcohols and Ethers172 Questions
Exam 12: Alcohols From Carbonyl Compounds Oxidation-Reduction and Organometallic Compounds147 Questions
Exam 13: Conjugated Unsaturated Systems166 Questions
Exam 14: Aromatic Compounds151 Questions
Exam 15: Reactions of Aromatic Compounds173 Questions
Exam 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group165 Questions
Exam 17: Aldehydes and Ketones Ii Aldol Reactions131 Questions
Exam 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon124 Questions
Exam 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions131 Questions
Exam 20: Amines148 Questions
Exam 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution87 Questions
Exam 22: Carbohydrates104 Questions
Exam 23: Lipids99 Questions
Exam 24: Amino Acids and Proteins94 Questions
Exam 25: Nucleic Acids and Protein Synthesis89 Questions
Select questions type
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 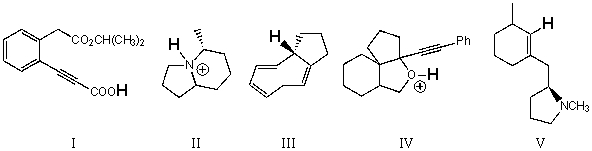
(Multiple Choice)
4.9/5  (39)
(39)
What does the reaction between the following two species produce? 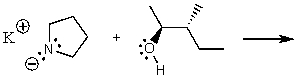
(Multiple Choice)
4.8/5  (36)
(36)
Which base would effectively deprotonate benzoic acid (PhCOOH)?
(Multiple Choice)
4.7/5  (42)
(42)
You are planning to carry out a reaction between propyne,CH3C≡CH and sodium amide,NaNH2.You also need to choose an appropriate solvent for carrying out the reaction.Would ethanol be suitable for this purpose? Explain your rationale clearly.
(Essay)
4.9/5  (31)
(31)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 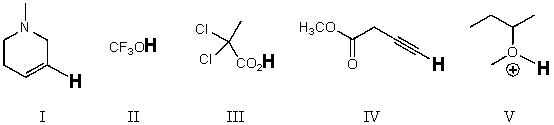
(Multiple Choice)
4.8/5  (37)
(37)
Which of the reaction conditions could afford the following transfromation? 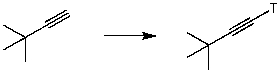
(Multiple Choice)
4.8/5  (30)
(30)
Which base would not effectively deprotonate phenol (PhOH)?
(Multiple Choice)
4.8/5  (44)
(44)
Briefly,but clearly,explain why the -OH hydrogen in acetic acid (CH3CO2H)is more acidic than in ethanol (C2H5OH).
(Essay)
4.7/5  (43)
(43)
What does the reaction between the following two species produce? 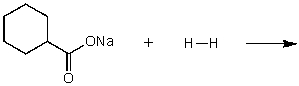
(Multiple Choice)
4.8/5  (38)
(38)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 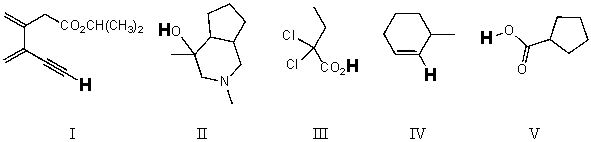
(Multiple Choice)
4.7/5  (34)
(34)
Bond polarization that takes place through space and through the bonds of the molecule is called the _____________.
(Short Answer)
4.7/5  (33)
(33)
Which of the reaction conditions would not afford the following transfromation? 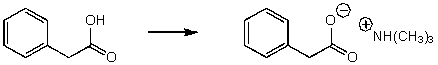
(Multiple Choice)
4.8/5  (39)
(39)
What is/are the product(s)of the following acid-base mechanism? 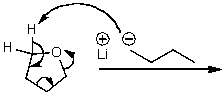
(Multiple Choice)
4.8/5  (33)
(33)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 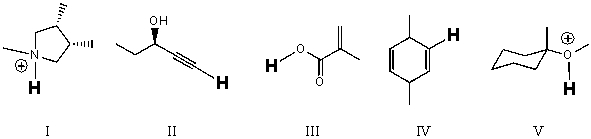
(Multiple Choice)
4.8/5  (34)
(34)
Which combination of reagents is the least effective in generating sodium isopropoxide, (CH3)2CH2ONa?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 47
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)