Exam 34: Wave Particle Duality and Quantum Physics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
The maximum kinetic energy of photoelectrons produced in the photoelectric effect depends directly on the
(Multiple Choice)
4.8/5  (39)
(39)
The dissociation energy is the energy required to separate the two atoms in a diatomic molecule in their ground-state.If the dissociation energy of molecular oxygen is 7.2 eV,then calculate the maximum wavelength of light from the Sun that can break apart atmospheric O2.
(Multiple Choice)
4.8/5  (43)
(43)
A proton has five times the momentum of an electron.If the electron has a de Broglie wavelength ,then the de Broglie wavelength of the proton is
(Multiple Choice)
4.9/5  (36)
(36)
The ground-state energy of hydrogen is -13.6 eV.The difference in energy between the n = 3 and n = 4 levels (magnitude only)is
(Multiple Choice)
4.9/5  (33)
(33)
The work function for tungsten is 4.58 eV.What is the kinetic energy of electrons emitted when light of wavelength 400 nm is incident on a tungsten surface? (Planck's constant h = 6.626 10-34 J · s = 4.136 10-15 eV · s.)
(Multiple Choice)
4.8/5  (30)
(30)
An electron has a kinetic energy of 1.5 eV.If its momentum is uncertain by ± 2.5%,the minimum uncertainty in its position is approximately
(Multiple Choice)
4.7/5  (37)
(37)
An electron is confined to a region of space of length L = 0.2 nm.Its minimum kinetic energy,assuming that p = p,is approximately
(Multiple Choice)
4.7/5  (34)
(34)
You want to make simultaneous measurements of the position and velocity of an electron that is moving in the positive-x direction.If the velocity is measured to an accuracy of 10-7 m/s,the limit of accuracy with which you can locate the electron is approximately
(Multiple Choice)
4.8/5  (34)
(34)
What is the momentum (in SI units)of a photon of wavelength = 410 nm? (Planck's constant h = 6.626 10-34 J·s)
(Multiple Choice)
4.8/5  (29)
(29)
Light falling on the surface of a metal such as cesium can liberate electrons from the metal.The kinetic energy of electrons emitted from a metal can be increased by
(Multiple Choice)
4.8/5  (37)
(37)
A classical point particle moves back and forth with constant speed between two walls at x = 0 and x = 4 cm.What is the probability of finding the particle between x = 3 cm and x = 3.4 cm?
(Multiple Choice)
4.8/5  (34)
(34)
The quantum theory suggests that the stable orbits of electrons around a nucleus correspond to standing waves for the orbit.For a hydrogen atom,the radius of the ground state or the first orbit is the Bohr's radius,0.0529 nm.What is the wavelength of the ground state of the hydrogen atom?
(Multiple Choice)
4.9/5  (29)
(29)
In a photoelectric experiment,the threshold frequency for a material is 3.2 1014 Hz.An electron ejected from this surface by a photon of frequency 9.4 1014 Hz can be stopped by a stopping potential of
(Multiple Choice)
4.8/5  (28)
(28)
Which of the following experiment(s)illustrates the particle nature of light?
(Multiple Choice)
4.9/5  (41)
(41)
The energy of the electron in a hydrogen atom in the ground state is -13.6 eV.If the potential is modeled like a one-dimensional box,the width of the box is
(Multiple Choice)
4.9/5  (32)
(32)
For an electron to have a de Broglie wavelength of 0.10 nm it must be accelerated through a potential difference of
(Multiple Choice)
4.8/5  (39)
(39)
Suppose an electron is confined in a one-dimensional box of width L which is two times the Bohr's radius of 0.0529 nm.What is the wavelength of the photon when the electron makes a transition from n = 3 to the ground state?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following particles has the longest wavelength?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements concerning particles and waves is/are correct?
(Multiple Choice)
4.9/5  (40)
(40)
Use the following figure to answer question. 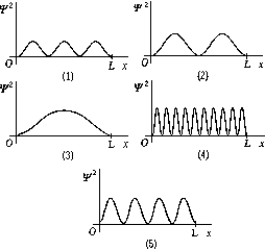 -The graphs show 2 as a function of x for a particle in a one-dimensional box of length L.The graph that represents the third excited state is
-The graphs show 2 as a function of x for a particle in a one-dimensional box of length L.The graph that represents the third excited state is
(Multiple Choice)
4.8/5  (38)
(38)
Showing 61 - 80 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)