Exam 34: Wave Particle Duality and Quantum Physics
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
An alpha particle (mass = 4 amu)is moving twice as fast as a proton (mass = 1 amu).Calculate the de Broglie wavelength of the proton divided by the de Broglie wavelength of the alpha particle.
(Multiple Choice)
4.9/5  (36)
(36)
A mass of 0.500 kg is oscillating with small amplitude oscillations on the end of a spring whose stiffness constant is 5 N/m.When this oscillator makes a transition from its n = 3 state to its n = 2 state,the energy of the photon emitted is
(Multiple Choice)
4.9/5  (36)
(36)
A proton is in a one-dimensional box of length 2 10-15 m.Find the wavelength of the photon emitted for a transition between the first excited state and the ground-state.
(Multiple Choice)
4.9/5  (32)
(32)
The wave-particle duality theory is the first adequate explanation of which one of the following observations about the hydrogen atom?
(Multiple Choice)
4.8/5  (42)
(42)
A gamma-ray photon of energy 100 keV scatters off an electron at an angle perpendicular to its original direction.Calculate the de Broglie wavelength of the recoiling electron.
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the photon energy for light of wavelength 500 nm.(Planck's constant h = 6.626 10-34 J·s)
(Multiple Choice)
4.8/5  (46)
(46)
Use the following equation statement to answer the question. The normalized wave functions for the infinite square-well potential are
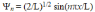 You may find it useful to use
You may find it useful to use
 -The expectation value <x> for a particle in the ground state of an infinite square-well potential is
-The expectation value <x> for a particle in the ground state of an infinite square-well potential is
(Multiple Choice)
4.8/5  (34)
(34)
The electron microscope is a welcome addition to the field of microscopy because electrons have a __________ wavelength than light,thereby increasing the __________ of the microscope.
(Multiple Choice)
4.9/5  (33)
(33)
Use the following figure to answer question. 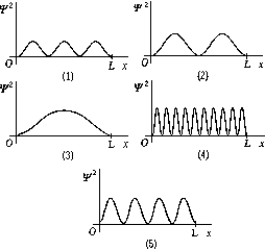 -The graphs show 2 as a function of x for a particle in a one-dimensional box of length L.The graph that represents the ground state is
-The graphs show 2 as a function of x for a particle in a one-dimensional box of length L.The graph that represents the ground state is
(Multiple Choice)
4.9/5  (33)
(33)
What is the shift in wavelength of a photon scattered off an electron at 160º?
(Multiple Choice)
4.7/5  (33)
(33)
An electron in the hydrogen atom (ground-state energy = -13.6 eV)makes a transition from the n = 3 to the n = 1 energy level.Calculate the magnitude of the energy of the photon involved in this process and state whether the photon was absorbed or emitted.
(Multiple Choice)
4.8/5  (33)
(33)
The wave function for a particle between x = -4 cm and 4 cm is given by 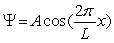 where L = 8 cm.Outside this range,the wave function is equal to zero.The probability of finding the particle between x = -2 cm and 2 cm is
where L = 8 cm.Outside this range,the wave function is equal to zero.The probability of finding the particle between x = -2 cm and 2 cm is
(Multiple Choice)
4.7/5  (31)
(31)
An electron is in a one-dimensional box of length 0.5 nm.The wavelength of the photon emitted as the electron transitions from its first excited state to its ground state is
(Multiple Choice)
4.9/5  (39)
(39)
Use the following equation statement to answer the question. The normalized wave functions for the infinite square-well potential are
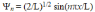 You may find it useful to use
You may find it useful to use
 -The expectation value <x> for a particle in the excited (n = 2)state of an infinite square-well potential is
-The expectation value <x> for a particle in the excited (n = 2)state of an infinite square-well potential is
(Multiple Choice)
4.8/5  (43)
(43)
The visible portion of the electromagnetic spectrum extends from
(Multiple Choice)
4.9/5  (32)
(32)
Microwaves range in wavelength from about 2.0 10-2 cm to about 5.0 cm.What is the maximum energy in eV that a microwave may have?
(Multiple Choice)
4.7/5  (28)
(28)
Photons of wavelength 0.00150 nm undergo Compton collisions with free electrons.What is the energy of the scattered photons whose angle of scattering is 45º?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 101 - 120 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)