Exam 36: Atoms
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
X rays are emitted when the electrons hit the screen in a cathode ray tube (CRT),i.e.,an old TV set.If the shortest wavelength X ray emitted is 3.7 10-2 nm,then what is the voltage that the electrons are accelerated through in the TV set?
(Multiple Choice)
4.7/5  (41)
(41)
According to Bohr's model,the radius of an electron at n = 4 is
(Multiple Choice)
4.9/5  (34)
(34)
A hydrogen atom is in an excited state having a binding energy of 0.85 eV in this state.The atom makes a transition to a state whose excitation energy is 10.2 eV.The energy of the photon emitted in this transition is approximately
(Multiple Choice)
4.9/5  (38)
(38)
An electron has a wave function given by 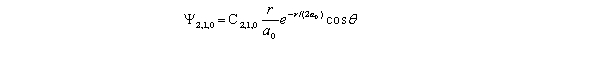 The probability of finding the electron when = 90 and = 0.5 ,and from r = a0 to 1.06a0 is approximately
The probability of finding the electron when = 90 and = 0.5 ,and from r = a0 to 1.06a0 is approximately
(Multiple Choice)
4.8/5  (39)
(39)
The first Bohr radius,r0,is 0.0529 nm and the corresponding energy,E0,is 13.6 eV.The wavelength of the light emitted as a hydrogen atom undergoes a transition from state n = 4 to n = 2 is
(Multiple Choice)
4.8/5  (36)
(36)
The order-of-magnitude of the diameter of the nucleus is closest to
(Multiple Choice)
5.0/5  (41)
(41)
For the hydrogen atom in the ground state,the wave function is 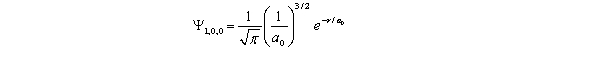 The probability of finding the electron from r = 0 to r = a0 is approximately Note:
The probability of finding the electron from r = 0 to r = a0 is approximately Note: 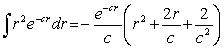
(Multiple Choice)
4.9/5  (32)
(32)
What is the wavelength of the most energetic X rays emitted from an X-ray tube operated at 40 kV?
(Multiple Choice)
4.9/5  (40)
(40)
The radius of the n = 1 orbit in the hydrogen atom is 0.053 nm.What is the radius of the n = 3 orbit of lithium,which has three protons in its nucleus?
(Multiple Choice)
4.8/5  (36)
(36)
The energy of an electron in an atom is determined primarily by the quantum numbers
(Multiple Choice)
4.9/5  (34)
(34)
The hydrogen-like elements (valence +1)exhibit a splitting of the energy states without an external magnetic field; this is due to
(Multiple Choice)
4.8/5  (43)
(43)
When the voltage across an X-ray tube is doubled,the wavelengths of the characteristic lines emitted by the tube
(Multiple Choice)
4.9/5  (43)
(43)
A compact disc of a CD player has a moment of inertia I = 2.5 10-5 kg · m2 and rotates at 500 rev/min.Taking L = I and using the quantization of angular momentum,the approximate value of L is ( 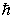 = 1.055 10-34 J · s)
= 1.055 10-34 J · s)
(Multiple Choice)
4.9/5  (37)
(37)
If the angular momentum is characterized by the quantum number 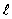 = 3,what is the largest possible angle between
= 3,what is the largest possible angle between 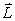 and the +z axis?
and the +z axis?
(Multiple Choice)
4.8/5  (35)
(35)
An electron in the n = 3 state of a hydrogen atom falls into the n = 2 state.The Rydberg constant R is 1.097 10-2/nm.The wavelength of the photon emitted is approximately
(Multiple Choice)
4.9/5  (28)
(28)
Consider a beryllium (Z = 4)ion with all but one of its electrons removed.What is the kinetic energy of the electron in the n = 1 orbit.(The radius of the 1st Bohr orbit in Be3+ is 0.0132 nm.)
(Multiple Choice)
4.7/5  (31)
(31)
The group of symbols that could represent the set of quantum numbers for a particular electron is
(Multiple Choice)
4.8/5  (28)
(28)
Showing 61 - 80 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)