Exam 36: Atoms
Exam 1: Systems of Measurement86 Questions
Exam 2: Motion in One Dimension83 Questions
Exam 3: Motion in Two and Three Dimensions60 Questions
Exam 4: Newtons Laws106 Questions
Exam 5: Applications of Newtons Laws73 Questions
Exam 6: Work and Energy60 Questions
Exam 7: Conservation of Energy56 Questions
Exam 8: Systems of Particles and Conservation of Linear Momentum92 Questions
Exam 9: Rotation105 Questions
Exam 10: Conservation of Angular Momentum66 Questions
Exam 11: Gravity84 Questions
Exam 12: Static Equilibrium and Elasticity58 Questions
Exam 13: Fluids77 Questions
Exam 14: Oscillations126 Questions
Exam 15: Wave Motion112 Questions
Exam 16: Superposition and Standing Waves87 Questions
Exam 17: Temperature and the Kinetic Theory of Gases78 Questions
Exam 18: Heat and the First Law of Thermodynamics100 Questions
Exam 19: The Second Law of Thermodynamics59 Questions
Exam 20: Thermal Properties and Processes50 Questions
Exam 21: The Electric Field I: Discrete Charge Distributions55 Questions
Exam 22: The Electric Field Ii: Continuous Charge Distributions64 Questions
Exam 23: Electric Potential87 Questions
Exam 24: Capacitance63 Questions
Exam 25: Electric Current and Direct-Current Circuits107 Questions
Exam 26: The Magnetic Field33 Questions
Exam 27: Sources of the Magnetic Field86 Questions
Exam 28: Magnetic Induction56 Questions
Exam 29: Alternating-Current Circuits106 Questions
Exam 30: Maxwells Equations and Electromagnetic Waves57 Questions
Exam 31: Properties of Light82 Questions
Exam 32: Optical Images106 Questions
Exam 33: Interference and Diffraction91 Questions
Exam 34: Wave Particle Duality and Quantum Physics140 Questions
Exam 35: Applications of the Schrodinger Equation42 Questions
Exam 36: Atoms113 Questions
Exam 37: Molecules39 Questions
Exam 38: Solids and the Theory of Conduction75 Questions
Exam 39: Relativity82 Questions
Exam 40: Nuclear Physics107 Questions
Exam 41: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
According to the Bohr theory,the allowed energy states for the hydrogen atom are given by the relation 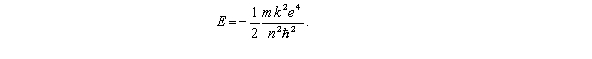 This formula can be readily extended to other hydrogenic (one-electron)systems.The energy of the second level (n = 2)for the doubly ionized lithium atom is
This formula can be readily extended to other hydrogenic (one-electron)systems.The energy of the second level (n = 2)for the doubly ionized lithium atom is
(Multiple Choice)
4.8/5  (36)
(36)
The number of electrons in the M shell for the element whose electronic configuration is 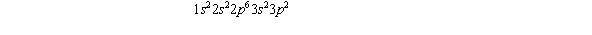 must be
must be
(Multiple Choice)
4.9/5  (44)
(44)
For the hydrogen atom in the ground state,the wave function is 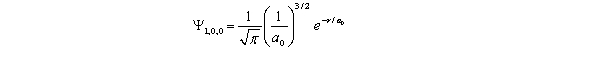 The probability of finding the electron from r = 0 to r = 2a0 is approximately Note:
The probability of finding the electron from r = 0 to r = 2a0 is approximately Note: 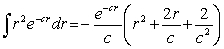
(Multiple Choice)
4.9/5  (33)
(33)
Evidence of electron capture by a nucleus would be the emission of
(Multiple Choice)
4.8/5  (27)
(27)
The kinetic energy of an electron moving in a circular orbit of radius r about a positive charge Ze varies
(Multiple Choice)
4.9/5  (46)
(46)
The limit (n = )of a particular spectral series is the line of
(Multiple Choice)
4.9/5  (34)
(34)
The energy of a hydrogen atom in the n = 5 state is approximately
(Multiple Choice)
4.9/5  (32)
(32)
The wavelength of the K X-ray line for an element is 0.0794 nm.The atomic number of the element is
(Multiple Choice)
4.7/5  (37)
(37)
The Pauli exclusion principle states that no two electrons in the same atom can have the same set of quantum numbers.This set of quantum numbers is
(Multiple Choice)
4.9/5  (36)
(36)
What is the energy difference between the transition with the longest wavelength in the Lyman series and the transition with the shortest wavelength in the Paschen series?
(Multiple Choice)
4.9/5  (36)
(36)
The wavelength of the photon emitted when a hydrogen atom undergoes a transition from the n = 5 state to the n = 1 state is approximately
(Multiple Choice)
4.8/5  (41)
(41)
When a gold foil is bombarded with alpha particles,a small fraction of the alpha particles were scattered at large angles.The large scattering angles
(Multiple Choice)
4.8/5  (35)
(35)
For the hydrogen atom in the ground state,the radial probability density is 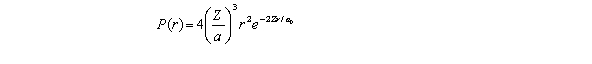 The probability of finding the electron in the range r = 0.04a0 at r = a0 is
The probability of finding the electron in the range r = 0.04a0 at r = a0 is
(Multiple Choice)
4.9/5  (33)
(33)
When the voltage across an X-ray tube is doubled,the minimum wavelength of the bremsstrahlung radiation
(Multiple Choice)
4.9/5  (40)
(40)
The number of electrons in the L shell for the element whose electronic configuration is  must be
must be
(Multiple Choice)
4.9/5  (39)
(39)
The wavelength of the visible line in the hydrogen spectrum that corresponds to m = 5 in the Balmer equation is
(Multiple Choice)
4.7/5  (45)
(45)
Showing 41 - 60 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)