Exam 15: Applications of the Schrodinger Equation
Exam 1: The Electric Field I: Discrete Charge Distributions87 Questions
Exam 2: The Electric Field II: Continuous Charge Distributions75 Questions
Exam 3: Electric Potential108 Questions
Exam 4: Capacitance73 Questions
Exam 5: Electric Current and Direct-Current Circuits160 Questions
Exam 6: The Magnetic Field71 Questions
Exam 7: Sources of the Magnetic Field115 Questions
Exam 8: Magnetic Induction84 Questions
Exam 9: Alternating-Current Circuits119 Questions
Exam 10: Maxwells Equations and Electromagnetic Waves61 Questions
Exam 11: Properties of Light116 Questions
Exam 12: Optical Images143 Questions
Exam 13: Interference and Diffraction116 Questions
Exam 14: Wave Particle Duality and Quantum Physics153 Questions
Exam 15: Applications of the Schrodinger Equation54 Questions
Exam 16: Atoms128 Questions
Exam 17: Molecules44 Questions
Exam 18: Solids and the Theory of Conduction83 Questions
Exam 19: Relativity83 Questions
Exam 20: Nuclear Physics135 Questions
Exam 21: Elementary Particles and the Beginning of the Universe68 Questions
Select questions type
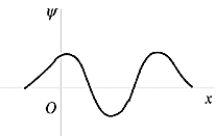 The wave function shown in the figure represents the n = _______ energy state of the harmonic oscillator.
The wave function shown in the figure represents the n = _______ energy state of the harmonic oscillator.
Free
(Multiple Choice)
5.0/5  (31)
(31)
Correct Answer:
C
An electron confined to a one-dimensional box of length L = 0.2 nm makes a transition from state n = 4 to state n = 3. The wavelength of the photon emitted is
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
The wave function for the energy level in a cubical box of side L that corresponds to the quantum numbers 1, 2, and 3 is
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
D
A particle is confined in a three-dimensional box with L1 = L2 = 3L3. The quantum numbers for the second excited state are
(Multiple Choice)
4.8/5  (36)
(36)
Particles that have antisymmetric wave functions and are described by the Pauli exclusion principle are called
(Multiple Choice)
4.9/5  (36)
(36)
 The graph that shows the second state for a particle in a finite square well is
The graph that shows the second state for a particle in a finite square well is
(Multiple Choice)
4.8/5  (37)
(37)
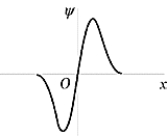 The wave function shown in the figure represents the n = _______ energy state of the harmonic oscillator.
The wave function shown in the figure represents the n = _______ energy state of the harmonic oscillator.
(Multiple Choice)
4.9/5  (34)
(34)
An electron of energy E0 traveling in a region in which the potential energy is zero is incident on a potential barrier of height U0 = 0.5E0. The ratio of the wavelength of the transmitted wave to the incident wave is
(Multiple Choice)
4.9/5  (34)
(34)
A particle of mass m is confined in a two-dimensional box that has sides Lx = L and Ly = 2L. By what factor is the energy of the 3rd excited state larger than the energy of the ground state?
(Multiple Choice)
4.9/5  (35)
(35)
The ground-state wave function of the harmonic oscillator is
(Multiple Choice)
4.9/5  (34)
(34)
An electron is confined in a two-dimensional box where U(x,y) = 0 for x = 0 to L and y = 0 to 3L, and U(x,y) = infinity outside these boundaries. If L = 0.5 nm, then calculate the energy of the first excited state.
(Multiple Choice)
4.8/5  (34)
(34)
An electron of kinetic energy E0 traveling in a region in which the potential energy is zero is then incident on a finite potential barrier of height U0 (= 4E0) and width a. If the potential barrier is reduced to 2E0, by what factor will the probability of penetration of the barrier be changed?
(Multiple Choice)
4.8/5  (29)
(29)
In order to solve the Schrödinger's equation, which of the following quantity(ies) must be specified?
(Multiple Choice)
4.8/5  (29)
(29)
You put 5 non-interacting identical fermions each of mass m into a 1-d box of dimension L. You then put 10 non-interacting bosons each of mass m into a 1-d box of length 2L. Which system has the lowest ground-state energy and what is the value of the fermion system ground-state energy divided by the boson system ground-state energy?
(Multiple Choice)
4.8/5  (40)
(40)
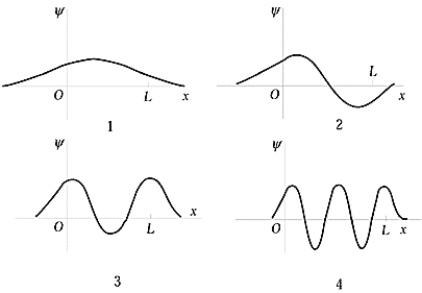
 The penetration of the wave function beyond the edges of the finite square-well potential shown in the figure
The penetration of the wave function beyond the edges of the finite square-well potential shown in the figure
(Multiple Choice)
4.8/5  (34)
(34)
The quantum phenomenon known as the "tunnel effect" refers to
(Multiple Choice)
4.9/5  (23)
(23)
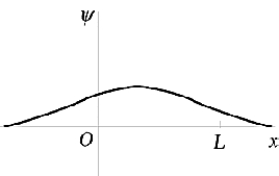
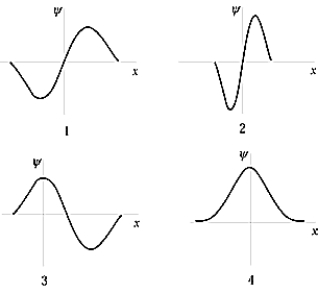 The ground-state wave function of the harmonic oscillator is best represented by
The ground-state wave function of the harmonic oscillator is best represented by
(Multiple Choice)
4.8/5  (31)
(31)
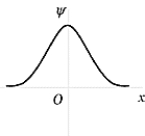 The wave function shown in the figure represents the n = _______ energy state of the harmonic oscillator.
The wave function shown in the figure represents the n = _______ energy state of the harmonic oscillator.
(Multiple Choice)
4.9/5  (22)
(22)
A particle is confined in a three-dimensional box with L1 = L, L2 = 2L and L3 = 3L. The energy levels of the particle are given by
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)