Exam 3: Acids and Bases
Exam 1: The Basics158 Questions
Exam 2: Families of Carbon151 Questions
Exam 3: Acids and Bases149 Questions
Exam 4: Nomenclature and Conformations of Alkanes and Cycloalkanes163 Questions
Exam 5: Stereochemistry179 Questions
Exam 6: Nucleophilic Reactions91 Questions
Exam 7: Alkenes and Alkynes I220 Questions
Exam 8: Alkenes and Alkynes II163 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry158 Questions
Exam 10: Radical Reactions148 Questions
Exam 11: Alcohols and Ethers256 Questions
Exam 12: Alcohols From Carbonyl Compounds210 Questions
Exam 13: Conjugated Unsaturated Systems201 Questions
Exam 14: Aromatic Compounds186 Questions
Exam 15: Reactions of Aromatic Compounds207 Questions
Exam 16: Aldehydes and Ketones189 Questions
Exam 17: Carboxylic Acids and Their Derivatives213 Questions
Exam 18: Reactions at the Α Carbon of Carbonyl Compounds190 Questions
Exam 19: Condensation and Conjugate Addition Reactions of Carbonyl Compounds182 Questions
Exam 20: Amines207 Questions
Exam 21: Transition Metal Complexes86 Questions
Exam 22: Carbohydrates124 Questions
Exam 23: Lipids127 Questions
Exam 24: Amino Acids and Proteins135 Questions
Exam 25: Nucleic Acids and Protein Synthesis126 Questions
Select questions type
The compound aniline,C6H5NH2,has weakly basic properties in aqueous solution.In this other solvent,aniline would behave as a strong base.
(Multiple Choice)
4.8/5  (37)
(37)
Based on the position of the central atom in the periodic chart,we predict that the strongest acid of the following is:
(Multiple Choice)
4.9/5  (34)
(34)
Why do water-insoluble carboxylic acids dissolve in aqueous sodium hydroxide?
(Essay)
4.7/5  (33)
(33)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 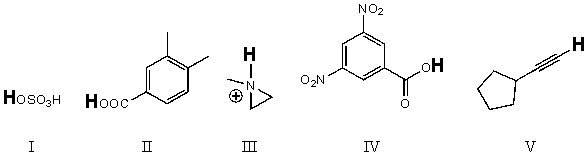
(Multiple Choice)
5.0/5  (27)
(27)
Which of the following substances has a hydrogen atom with pKa ≈ 25?
(Multiple Choice)
4.9/5  (32)
(32)
Briefly,but clearly,explain why the -OH hydrogen in acetic acid (CH3CO2H)is more acidic than in ethanol (C2H5OH).
(Essay)
4.9/5  (34)
(34)
Which of the reaction conditions could afford the following transformation? 
(Multiple Choice)
4.8/5  (38)
(38)
In the following reaction which chemical species is acting like a nucleophile? 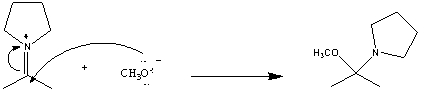
(Multiple Choice)
4.9/5  (33)
(33)
A group of acids arranged in order of decreasing acidity is: HNO3 > CH3COOH > C6H5OH > H2O > HC CH
What is the arrangement of the conjugate bases of these compounds in decreasing order of basicity?
(Multiple Choice)
4.8/5  (32)
(32)
For a reaction that has positive change in entropy,increasing the temperature of the reaction will help make the reaction energetically favorable to form products.
(True/False)
4.9/5  (29)
(29)
An acid,HA,has the following thermodynamic values for its dissociation in water at 27 ºC: H = -8.0 kJ mol-1; S = -70 J K-1mol-1.The G for the process is:
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is not a conjugate acid - conjugate base pair (in that order)?
(Multiple Choice)
4.9/5  (39)
(39)
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 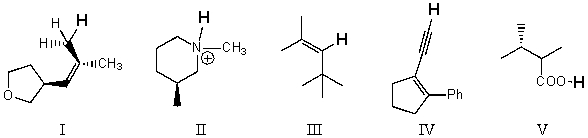
(Multiple Choice)
4.8/5  (37)
(37)
Showing 61 - 80 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)