Exam 15: Reactions of Aromatic Compounds
Exam 1: The Basics158 Questions
Exam 2: Families of Carbon151 Questions
Exam 3: Acids and Bases149 Questions
Exam 4: Nomenclature and Conformations of Alkanes and Cycloalkanes163 Questions
Exam 5: Stereochemistry179 Questions
Exam 6: Nucleophilic Reactions91 Questions
Exam 7: Alkenes and Alkynes I220 Questions
Exam 8: Alkenes and Alkynes II163 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry158 Questions
Exam 10: Radical Reactions148 Questions
Exam 11: Alcohols and Ethers256 Questions
Exam 12: Alcohols From Carbonyl Compounds210 Questions
Exam 13: Conjugated Unsaturated Systems201 Questions
Exam 14: Aromatic Compounds186 Questions
Exam 15: Reactions of Aromatic Compounds207 Questions
Exam 16: Aldehydes and Ketones189 Questions
Exam 17: Carboxylic Acids and Their Derivatives213 Questions
Exam 18: Reactions at the Α Carbon of Carbonyl Compounds190 Questions
Exam 19: Condensation and Conjugate Addition Reactions of Carbonyl Compounds182 Questions
Exam 20: Amines207 Questions
Exam 21: Transition Metal Complexes86 Questions
Exam 22: Carbohydrates124 Questions
Exam 23: Lipids127 Questions
Exam 24: Amino Acids and Proteins135 Questions
Exam 25: Nucleic Acids and Protein Synthesis126 Questions
Select questions type
Consider the structures given below.Which of them would be a relatively stable contributor to the hybrid formed when toluene undergoes para bromination? 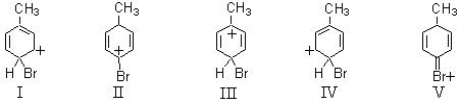
(Multiple Choice)
4.9/5  (45)
(45)
Which reagent would you use to carry out the following transformation? tert-butylbenzene p-tert-butylbenzenesulfonic acid
+
O-tert-butylbenzenesulfonic acid
(Multiple Choice)
4.8/5  (36)
(36)
Which of these is the rate-determining step in the bromination of benzene?
(Multiple Choice)
4.8/5  (29)
(29)
What would you expect to be the major product obtained from the following reaction? 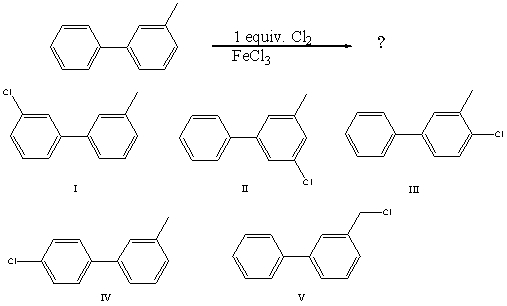
(Multiple Choice)
4.9/5  (29)
(29)
Based on your knowledge of electrophilic aromatic substitution,predict the preferential position of nitration of naphthalene,i.e.,does the NO2+ attack carbon 1,or 2? Explain your answer. 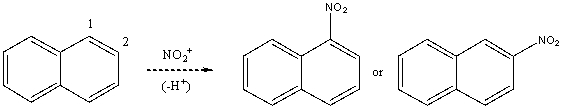 Which substitution product forms preferentially and why?
Which substitution product forms preferentially and why?
(Essay)
4.8/5  (46)
(46)
Which would be the product of the following reaction sequence? 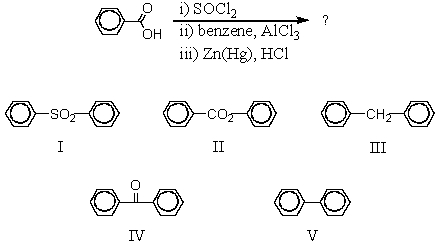
(Multiple Choice)
4.9/5  (39)
(39)
Using a potential energy diagram,explain/illustrate the preferential formation of the 3 substituted product instead of the 2 product in the electrophilic aromatic substitution of benzo[b] furane (shown). ![Using a potential energy diagram,explain/illustrate the preferential formation of the 3 substituted product instead of the 2 product in the electrophilic aromatic substitution of benzo[b] furane (shown).](https://storage.examlex.com/TB5902/11eaa4bc_3e01_222b_9180_774b12a0342d_TB5902_00.jpg)
(Essay)
4.7/5  (44)
(44)
What would you expect to be the major product obtained from the following reaction? 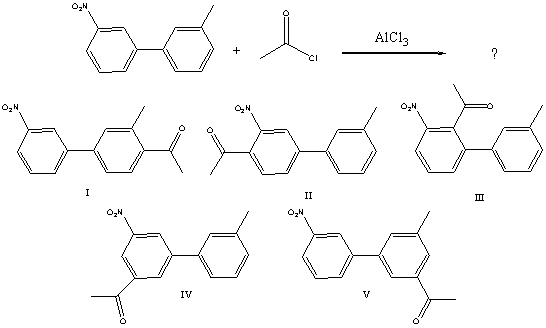
(Multiple Choice)
4.9/5  (43)
(43)
The ortho/para product ratio is expected to be the smallest for the bromination of which of these?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following is not a meta-directing substituent when present on the benzene ring?
(Multiple Choice)
4.7/5  (34)
(34)
Which is the best sequence of reactions for the following transformation? 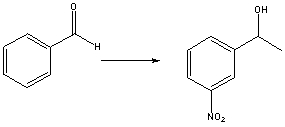
(Multiple Choice)
4.7/5  (40)
(40)
What would you expect to be the major product obtained from the monobromination of m-dichlorobenzene? 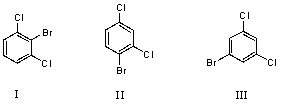
(Multiple Choice)
4.8/5  (35)
(35)
The compound 4-bromo-1-propylbenzene is best made from benzene by the application of these reagents in the order shown:
(Multiple Choice)
4.8/5  (35)
(35)
Each of the five disubstituted benzenes shown below is nitrated.In which of these cases does the arrow not indicate the chief position of nitration. 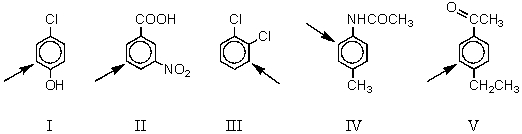
(Multiple Choice)
4.7/5  (36)
(36)
Showing 181 - 200 of 207
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)