Exam 15: Reactions of Aromatic Compounds
Exam 1: The Basics158 Questions
Exam 2: Families of Carbon151 Questions
Exam 3: Acids and Bases149 Questions
Exam 4: Nomenclature and Conformations of Alkanes and Cycloalkanes163 Questions
Exam 5: Stereochemistry179 Questions
Exam 6: Nucleophilic Reactions91 Questions
Exam 7: Alkenes and Alkynes I220 Questions
Exam 8: Alkenes and Alkynes II163 Questions
Exam 9: Nuclear Magnetic Resonance and Mass Spectrometry158 Questions
Exam 10: Radical Reactions148 Questions
Exam 11: Alcohols and Ethers256 Questions
Exam 12: Alcohols From Carbonyl Compounds210 Questions
Exam 13: Conjugated Unsaturated Systems201 Questions
Exam 14: Aromatic Compounds186 Questions
Exam 15: Reactions of Aromatic Compounds207 Questions
Exam 16: Aldehydes and Ketones189 Questions
Exam 17: Carboxylic Acids and Their Derivatives213 Questions
Exam 18: Reactions at the Α Carbon of Carbonyl Compounds190 Questions
Exam 19: Condensation and Conjugate Addition Reactions of Carbonyl Compounds182 Questions
Exam 20: Amines207 Questions
Exam 21: Transition Metal Complexes86 Questions
Exam 22: Carbohydrates124 Questions
Exam 23: Lipids127 Questions
Exam 24: Amino Acids and Proteins135 Questions
Exam 25: Nucleic Acids and Protein Synthesis126 Questions
Select questions type
Based on your knowledge of electrophilic aromatic substitution,and assuming the furan ring of benzo[b]furan (shown)is activated toward electrophilic aromatic substituted more so than the benzenoid ring,predict the preferential position of attack of an electrophile,i.e.,does the electrophile E+ attack carbon 2,or 3? Explain your answer. ![Based on your knowledge of electrophilic aromatic substitution,and assuming the furan ring of benzo[b]furan (shown)is activated toward electrophilic aromatic substituted more so than the benzenoid ring,predict the preferential position of attack of an electrophile,i.e.,does the electrophile E<sup>+</sup> attack carbon 2,or 3? Explain your answer. Which substitution product forms preferentially and why?](https://storage.examlex.com/TB5902/11eaa4bc_3e00_d403_9180_31f378e6d8d5_TB5902_00.jpg) Which substitution product forms preferentially and why?
Which substitution product forms preferentially and why?
(Essay)
4.7/5  (29)
(29)
Which of the following is not an ortho-para director in electrophilic aromatic substitution?
(Multiple Choice)
4.8/5  (39)
(39)
Using a potential energy diagram,explain/illustrate the preferential formation of the 3 substituted product instead of the 2 or 4 product in the electrophilic aromatic substitution of pyridine. 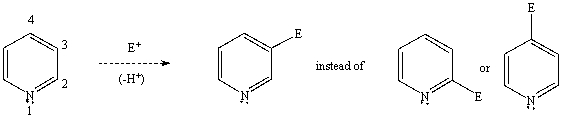
(Essay)
4.7/5  (38)
(38)
Which reagent(s)would you use to carry out the following transformation? toluene benzoic acid
(Multiple Choice)
4.8/5  (34)
(34)
Based on your knowledge of electrophilic aromatic substitution,and assuming the pyrrole ring of indole (shown)is activated toward electrophilic aromatic substituted more so than the benzenoid ring,predict the preferential position of attack of an electrophile,i.e.,does the electrophile E+ attack carbon 2,or 3? Explain your answer. 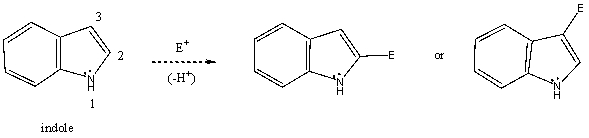 Which substitution product forms preferentially and why?
Which substitution product forms preferentially and why?
(Essay)
4.9/5  (32)
(32)
Based on your knowledge of electrophilic aromatic substitution,predict the preferential position of attack of an electrophile on pyrrole,i.e.,does the electrophile E+ attack carbon 2,or 3? Explain your answer. 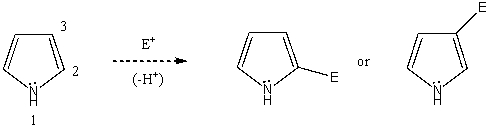 Which substitution product forms preferentially and why?
Which substitution product forms preferentially and why?
(Essay)
4.9/5  (31)
(31)
The reaction of benzene with (CH3)3CCH2Cl in the presence of anhydrous aluminum chloride produces principally which of these? 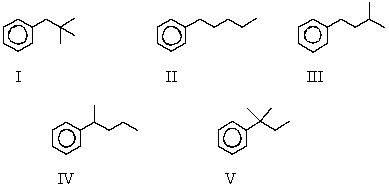
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following is not an ortho-para director in electrophilic aromatic substitution?
(Multiple Choice)
4.8/5  (36)
(36)
What is the chief product of the Friedel-Crafts alkylation of benzene with 1-butene and HF?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following structures contribute(s)to the resonance hybrid of the intermediate formed when acetanilide undergoes para-bromination? 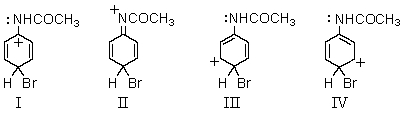
(Multiple Choice)
4.9/5  (23)
(23)
When two different groups are present on a benzene ring,the ___ generally determines the outcome of an EAS reaction.
(Short Answer)
4.8/5  (37)
(37)
Consider the resonance forms shown for the arenium ionformed from the bromination of acetanilide.Which resonance form contributes most to the overall resonance hybrid? 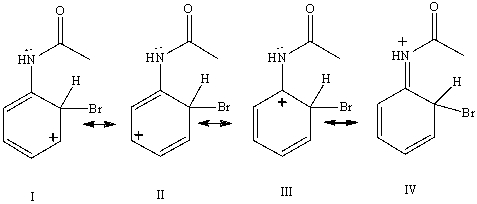
(Multiple Choice)
4.7/5  (36)
(36)
Draw a mechanism that explains the formation of the following product in this Friedel-Crafts alkylation: 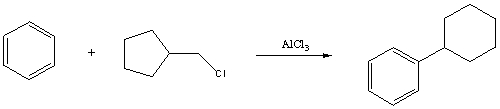
(Essay)
4.7/5  (42)
(42)
The electrophilic bromination or chlorination of benzene requires,in addition to the halogen:
(Multiple Choice)
4.7/5  (30)
(30)
Acid-catalyzed hydration of 1-phenyl-1-pentene gives 1-phenyl-1-pentanol almost exclusively; the other possible hydration product,1-phenyl-2-pentanol,is not detected at all.Explain clearly.
(Essay)
4.9/5  (35)
(35)
What would you expect to be the major product obtained from the following reaction? 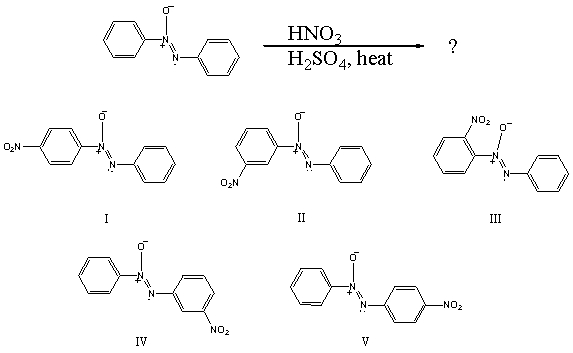
(Multiple Choice)
5.0/5  (42)
(42)
Showing 101 - 120 of 207
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)