Exam 14: Acids and Bases
Exam 1: Chemical Foundations96 Questions
Exam 2: Atoms, molecules, and Ions91 Questions
Exam 3: Stoichiometry134 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry117 Questions
Exam 5: Gases132 Questions
Exam 6: Thermochemistry86 Questions
Exam 7: Atomic Structure and Periodicity149 Questions
Exam 8: Bonding: General Concepts146 Questions
Exam 9: Covalent Bonding: Orbitals101 Questions
Exam 10: Liquids and Solids126 Questions
Exam 11: Properties of Solutions108 Questions
Exam 12: Chemical Kinetics113 Questions
Exam 13: Chemical Equilibrium87 Questions
Exam 14: Acids and Bases149 Questions
Exam 15: Acid-Base Equilibria94 Questions
Exam 16: Solubility and Complex Ion Equilibria93 Questions
Exam 17: Spontaneity, entropy, and Free Energy117 Questions
Exam 18: Electrochemistry138 Questions
Exam 19: The Nucleus: a Chemists View83 Questions
Exam 20: The Representative Elements154 Questions
Exam 21: Transition Metals and Coordination Chemistry142 Questions
Exam 22: Organic and Biological Molecules164 Questions
Select questions type
What is the equilibrium pH of a 0.835 M solution of H3PO4(aq)? (Ka1 = 7.5 10-3,Ka2 = 6.2 10-8,Ka3 = 4.8 10-13)
(Multiple Choice)
4.8/5  (32)
(32)
The equilibrium constants (Ka)for HCN and HF in H2O at 25°C are 6.2 10-10 and 7.2 10-4,respectively.The relative order of base strengths is:
(Multiple Choice)
4.9/5  (36)
(36)
At 65°C,the ion-product constant of water,Kw,is 1.20 10-13.The pH of pure water at 65°C is:
(Multiple Choice)
4.8/5  (38)
(38)
Determine whether the following oxides produce an acidic,basic,or neutral solution when dissolved in water:
-Cl2O
(Essay)
4.7/5  (36)
(36)
A solution of 8.01 M formic acid (HCOOH)is 0.47% ionized.What is the Ka value of formic acid?
(Multiple Choice)
4.9/5  (40)
(40)
Select the answer that best describes an aqueous solution made from each of the following substances:
-solid aluminum chloride (AlCl3)
(Multiple Choice)
4.9/5  (41)
(41)
The [H3O+] of a 0.49 M solution of NH4Cl in H2O at 25°C is (Kb for NH3 = 1.8 10-5):
(Multiple Choice)
4.9/5  (37)
(37)
Given the following acids and Ka values:
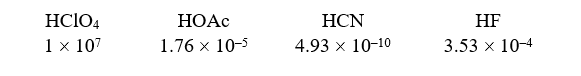 What is the order of increasing base strength?
What is the order of increasing base strength?
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the pH of the following aqueous solution: 0.39 M NH4Cl (pKb for NH3 = 4.74)
(Multiple Choice)
4.9/5  (43)
(43)
Carbonic acid is a diprotic acid,H2CO3,with Ka1 = 4.2 10-7 and Ka2 = 4.8 10-11 at 25°C.The ion product for water is Kw = 1.0 10-14 at 25°C.What is the OH- concentration of a solution that is 0.18 M in Na2CO3?
(Multiple Choice)
4.9/5  (42)
(42)
Explain why Al2(SO4)3 produces an acidic solution when it is dissolved in water.
(Essay)
4.9/5  (33)
(33)
Calculate the pH of the following aqueous solution: 0.66 M HOCl (pKa = 7.46)
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the [H+] in a 0.068 M solution of HCN,Ka = 6.2 10-10.
(Multiple Choice)
4.9/5  (47)
(47)
Calculate the pH of a 0.005 M solution of potassium oxide,K2O.
(Multiple Choice)
4.7/5  (33)
(33)
Calculate the pH of a 0.59 M solution of NH4Cl.(Kb for NH3 = 1.8 10-5)
(Multiple Choice)
4.8/5  (35)
(35)
Consider the following reactions:
a)Al3+ + 6H2O  Al(OH2)63+
b)Al(OH2)63+
Al(OH2)63+
b)Al(OH2)63+  Al(OH)(OH2)52+ + H+
c)OCl- + H2O
Al(OH)(OH2)52+ + H+
c)OCl- + H2O  HOCl + OH-
d)CN- + H+
HOCl + OH-
d)CN- + H+  HCN
e)none of these
-Which is associated with the definition of Ka?
HCN
e)none of these
-Which is associated with the definition of Ka?
(Multiple Choice)
4.7/5  (32)
(32)
What concentration of acetic acid (Ka = 1.80 10-5)has the same pH as that of 5.33 10-3 M HCl?
(Multiple Choice)
4.8/5  (31)
(31)
For the stepwise dissociation of aqueous H3PO4,which of the following is not a conjugate acid-base pair?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 149
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)