Exam 2: Atoms and the Atomic Theory
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
A certain element contains eleven atoms of mass 95.952 u for every four atoms of mass 98.949 u. Compute the average atomic weight of this element.
(Multiple Choice)
5.0/5  (34)
(34)
When decomposed chemically, 73.0 grams of a sample of HCl produce 71.0 g of Cl2 and 2.0 g of H2, while 34.0 g of a sample of H2S produce 32.0 g of S and 2.0 g of H2. This is an example of the Law:
(Multiple Choice)
4.8/5  (48)
(48)
Which of the following is a correct feature of the nuclear atom proposed by Rutherford?
(Multiple Choice)
4.8/5  (38)
(38)
Lead-204 has a relative abundance of 1.48%. What size block of lead (in cubic centimeters) will contain 8.34 × 1021 atoms of lead-204? The density of lead is 11.35 g/cm3.
(Multiple Choice)
4.8/5  (39)
(39)
The following ratios of masses were obtained with a mass spectrometer: 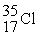 /
/ 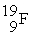 = 1.8406,
= 1.8406, 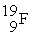 /
/ 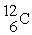 = 1.5832 What is the mass of a
= 1.5832 What is the mass of a 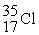 atom in atomic mass units?
atom in atomic mass units?
(Multiple Choice)
4.8/5  (37)
(37)
How many Cu atoms are present in a 75.0 cm length of 20-gauge copper wire? A 20-gauge wire has a diameter of 0.03196 in. Copper's density is 8.92 g/cm3.
(Multiple Choice)
4.9/5  (39)
(39)
Dalton's atomic theory is based on several assumptions, which are listed below. Which of these assumptions is strictly correct? I) All atoms of the same element are identical.
II) Atoms are indivisible and unchangeable.
III) Chemical changes are the result of the combination, separation, and rearrangement of atoms.
(Multiple Choice)
4.8/5  (34)
(34)
How many atoms of rubidium-85 are in 87.2 g of rubidium? Rubidium-85 is 72.2 % abundant.
(Multiple Choice)
4.8/5  (31)
(31)
An isotope with mass number 81 has eleven more neutrons than protons. This is an isotope of what element?
(Multiple Choice)
4.8/5  (37)
(37)
Ernest Rutherford is credited with: I) the nuclear model of the atom
II) the identification of alpha and beta particles
III) the discovery of protons
IV) the prediction of a third, neutral, subatomic particle
(Multiple Choice)
5.0/5  (44)
(44)
Which of the following would be unaffected by an electric field?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements is true concerning the masses of individual Cl atoms?
(Multiple Choice)
4.8/5  (39)
(39)
Copper occurs in an isotopic mixture of 69.09% 63Cu (mass = 62.93 u per atom) and 30.91% 65Cu (mass = 64.93 u per atom). What is the average atomic mass of copper?
(Multiple Choice)
4.8/5  (36)
(36)
What is the isotopic atomic mass of an isotope if 9.7023 × 1022 atoms weighs 4.0256 g?
(Multiple Choice)
5.0/5  (39)
(39)
How many atoms of hydrogen are present in 1.5 lb of hydrogen peroxide, which is 5.93% hydrogen? (1 lb = 454 grams)
(Multiple Choice)
4.7/5  (37)
(37)
Showing 21 - 40 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)