Exam 2: Atoms and the Atomic Theory
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
The average atomic mass of B is 10.80 u. Boron has only two stable forms 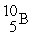 (10.00 u) and
(10.00 u) and 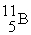 (11.00 u). What is the natural percent abundance of
(11.00 u). What is the natural percent abundance of 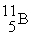 ?
?
(Multiple Choice)
4.8/5  (36)
(36)
A sample of pure carbon weighing 1.48 g was burned in an excess of air. The mass of carbon dioxide, the sole product, was 5.42 g. In a second experiment, 11.62 g of carbon dioxide was obtained. What mass of carbon was burned in the second experiment?
(Multiple Choice)
4.8/5  (35)
(35)
A hypothetical element, E, has two stable isotopes: E-46 = 46.046 u 64.08% E-51 = 50.826 u 35.92%
What is the average atom weight of the element?
(Multiple Choice)
4.9/5  (33)
(33)
An element has 5 stable isotopes. The mass and percentage of each are: 89.9043 51.46%
90)9053 11.23%
91)9046 17.11%
93)9061 17.40%
95)9082 2.80%
The element is which of the following?
(Multiple Choice)
4.8/5  (40)
(40)
What is the mass number of the most abundant form of oxygen atom?
(Multiple Choice)
4.8/5  (34)
(34)
When a chemical reaction is carried out in a sealed container, the substances may change in color, temperature, or state, but no change in mass is detected. This is evidence of the:
(Multiple Choice)
4.7/5  (39)
(39)
A cubic centimeter of lead weighs 11.35 g. How many atoms are in the block?
(Multiple Choice)
4.8/5  (36)
(36)
A 1.4 g sample of calcium is reacted with 3.2 g of oxygen. The only product after the reaction is 1.96 g of CaO. How many grams of oxygen remains unreacted?
(Multiple Choice)
5.0/5  (44)
(44)
Which of these atoms has the greatest number of neutrons in its nucleus?
(Multiple Choice)
4.9/5  (34)
(34)
What is the proper 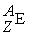 notation for an ion having 35 protons, 36 electrons and 45 neutrons?
notation for an ion having 35 protons, 36 electrons and 45 neutrons?
(Multiple Choice)
4.8/5  (26)
(26)
Rubidium possesses two stable forms and has an average mass of 85.5. 85Rb has a mass of 84.9 and a percent abundance of 72.2%. What is the mass of the other form of Rb?
(Multiple Choice)
4.9/5  (43)
(43)
Write the symbol for the radioactive isotope phosphorus-32.
(Multiple Choice)
4.9/5  (32)
(32)
A new element is discovered. It has two isotopes. The relative abundance of the isotopes and their masses are 18% isotope 1, mass 350.0 u and 82% isotope 2, mass 352.0 u. What is the atomic mass of the element?
(Multiple Choice)
4.8/5  (41)
(41)
A cation has 28 neutrons and 21 electrons. If it has a +3 charge, what is its correct symbol?
(Multiple Choice)
4.8/5  (34)
(34)
Silver possesses two stable isotopes: 107Ag (106.90 u) and 109Ag (108.90 u). If the average atomic mass of Ag is 107.87 u, what is the percent abundance of 107Ag?
(Multiple Choice)
4.9/5  (39)
(39)
What is the thickness in mm of the sheet 75.0 cm × 35.0 cm formed by 7.88 × 1023 atoms of lead? The density of lead is 11.35 g/cm3.
(Multiple Choice)
4.7/5  (39)
(39)
A solution contains 12.5% NaCl by mass. What mass of solution is required to obtain 1.0 × 1023 Na atoms?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 81 - 100 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)