Exam 1: Matter - Its Properties and Measurement
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
What is the mass of a 468 mL sample of ethanol? The density of ethanol is 0.789 g/mL.
(Multiple Choice)
4.7/5  (36)
(36)
What is the answer to the correct number of significant figures of the following calculation? 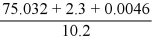
(Multiple Choice)
4.8/5  (30)
(30)
What mass, in g, of a solution containing 12% by mass sodium chloride is needed for a process that requires 8 g of sodium chloride?
(Multiple Choice)
4.9/5  (42)
(42)
If gasoline costs 98.5 cents per liter in Canada, what is the price in dollars per gallon?
(Multiple Choice)
4.8/5  (36)
(36)
The quantity of matter in an object is the object's ________ and is measured on a ________.
(Multiple Choice)
4.9/5  (39)
(39)
A briefcase 4.5 in × 22 in × 11 in is filled with gold, which has a density of 19.3 g/cm3 and costs $308/oz. What is the mass of this gold in kg?
(Multiple Choice)
5.0/5  (28)
(28)
What is the answer to the correct number of significant figures of the following calculation? 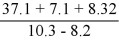
(Multiple Choice)
4.8/5  (26)
(26)
Ethylene glycol has a density of 1.11 g/mL. What is the volume occupied by 30.0 g of ethylene glycol?
(Multiple Choice)
4.9/5  (38)
(38)
From ammonia gas, one can obtain two different gases, each of which is a pure substance. Using only this information, it can be said with certainty that:
(Multiple Choice)
4.9/5  (33)
(33)
What is the answer to the correct number of significant figures of the following calculation? 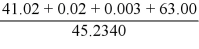
(Multiple Choice)
4.7/5  (40)
(40)
The composition refers to the components of a sample of matter and their relative proportions.
(True/False)
4.9/5  (41)
(41)
The result of addition can have more significant figures than any of the numbers added.
(True/False)
4.9/5  (36)
(36)
The content of a container filled with sand and water would best be described as:
(Multiple Choice)
4.9/5  (38)
(38)
What is the answer, to the correct number of significant figures, of the following calculation? 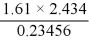
(Multiple Choice)
4.8/5  (45)
(45)
Systematic errors are consequence of inherent errors in measuring devices.
(True/False)
4.9/5  (28)
(28)
Showing 41 - 60 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)