Exam 1: Matter - Its Properties and Measurement
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
A physical property is the ability of a sample of matter to undergo a change in composition under certain conditions.
(True/False)
4.8/5  (35)
(35)
In Europe, the volume of soda is given in cL. How many mL are contained in a can of soda labeled "75 cL"?
(Multiple Choice)
5.0/5  (34)
(34)
A model that explains and makes predictions about natural phenomena is referred to as a:
(Multiple Choice)
4.9/5  (39)
(39)
At what temperature will the numerical values of the Celsius and Fahrenheit scales be the same?
(Multiple Choice)
4.9/5  (31)
(31)
The following measurements were made by a group of students using the same balance and a 25.00 gram weight. 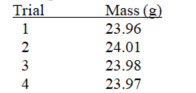 The data would be considered:
I.accurate and precise
II. accurate but not precise
III. precise but not accurate
IV. neither precise nor accurate
V. evidence of a systematic error
VI. evidence of large random errors
The data would be considered:
I.accurate and precise
II. accurate but not precise
III. precise but not accurate
IV. neither precise nor accurate
V. evidence of a systematic error
VI. evidence of large random errors
(Multiple Choice)
5.0/5  (33)
(33)
On a very cold day in Alaska, the temperature was -40°F. The temperature in Celsius on the same day was also -40°.
(True/False)
4.7/5  (36)
(36)
Which of the following are forms of matter?
I. hydrogen gas
II. sunlight
III. ice
IV. wind
V. iron
(Multiple Choice)
4.8/5  (34)
(34)
What is 21.1 miles/hour in furlongs/fortnight? (8 furlongs = 1 mile; 14 days = 1 fortnight)
(Multiple Choice)
4.8/5  (33)
(33)
A sprinter runs exactly 200 meters in 19.98 seconds. What is his/her average speed in miles per hour?
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following would be classified as a heterogeneous mixture?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the length of a steel cable (density 7.91 g/cm3) with a mass of 160.0 g and cross-sectional area of 0.500 cm2.
(Multiple Choice)
4.9/5  (39)
(39)
Showing 81 - 94 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)