Exam 17: Properties of Solutions
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
A solution containing 100.0 g of NaCl per liter has a density of 1.095 g/mL. What is the molality of the solution?
(Multiple Choice)
4.8/5  (33)
(33)
Pentane (C5H12) and hexane (C6H14) form an ideal solution. The vapor pressures of pentane and hexane at 25° C are 511 torr and 150 torr, respectively. The mole fraction of hexane in a pentane-hexane solution is 0.50. Calculate the mole fraction of pentane in the vapor that is in equilibrium at 25°C with this solution.
(Multiple Choice)
4.7/5  (36)
(36)
Consider that 45.0 g of a protein has been dissolved in 1.00 L of aqueous solution. The osmotic pressure of the solution at 25°C is 12.7 torr. What is the approximate molar mass of the protein?
(Short Answer)
4.9/5  (44)
(44)
When a nonvolatile solute is added to a volatile solvent, the solution vapor pressure __________, the boiling point __________, the freezing point __________, and the osmotic pressure across a semipermeable membrane __________.
(Multiple Choice)
4.9/5  (27)
(27)
At a given temperature, you have a mixture of benzene (vapor pressure of pure benzene = 745 torr) and toluene (vapor pressure of pure toluene = 290 torr). The mole fraction of benzene in the vapor above the solution is 0.590. Assuming ideal behavior, calculate the mole fraction of toluene in the solution.
(Multiple Choice)
4.8/5  (37)
(37)
When a 1.50-g sample of glutamic acid is dissolved in 100.0 g of H2O, the resulting solution freezes at -0.190°C. Kf for H2O is 1.86°C/m. The molar mass of glutamic acid is
(Multiple Choice)
4.8/5  (36)
(36)
When 0.014 mol of a weak acid HA is dissolved in enough water to make 1.0 L of solution, [H+] is 1.8 10-3 M. Calculate the freezing point of this solution. Assume that the molarity concentration is equal to the molality concentration, and assume ideal behavior. Kf (H2O) is 1.86°C kg/mol.
(Multiple Choice)
4.8/5  (37)
(37)
Rank the following compounds according to increasing solubility in water.
I.CH3-CH2-CH2-CH3
II.CH3-CH2-O-CH2-CH3
III. CH3-CH2-OH
IV. CH3-OH
(Multiple Choice)
4.8/5  (36)
(36)
What is the molar mass of glucose if 22.5 g gives a freezing point of -0.930°C when dissolved in 250.0 g of water? If the empirical formula is CH2O, what is the molecular formula?
(Short Answer)
4.9/5  (34)
(34)
The freezing-point depression of a 3.00 *10-3 M solution of a weak acid HA is 6.67* 10-3 °C in water. Assuming that the molarity concentration and the molality concentration are the same and assuming ideal behavior, calculate Ka for the weak acid. Kf(H2O) = 1.86 °C kg/mol.
(Short Answer)
4.9/5  (33)
(33)
Predict the deviation from Raoult's law when two liquids are mixed and the heat of the solution is small.
(Multiple Choice)
4.9/5  (39)
(39)
When 271.0 mL of 0.828 M HCl is diluted with 161.6 mL of water, the molarity of the solution (assuming the volumes are additive) is
(Multiple Choice)
4.8/5  (34)
(34)
The molal freezing-point depression constants for benzene and water are 5.12 and 1.86, respectively. When 4.6 g of formic acid (HCOOH) is dissolved in 1.0 kg of benzene, the freezing point is depressed by 0.26°C. When the same amount of formic acid is dissolved in 1.0 kg of water, the freezing point is lowered by 0.19°C. To explain these results, we must assume that
(Multiple Choice)
4.7/5  (38)
(38)
Acetone (mw = 58.08, P 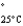 =232 mmHg) and butanone (mw = 72.11, P
=232 mmHg) and butanone (mw = 72.11, P 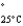 =100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
=100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
(Multiple Choice)
4.8/5  (45)
(45)
Diagram and label a vapor pressure diagram for an ideal solution of two volatile liquids. Indicate the deviation predicted by an endothermic heat of solution.
(Essay)
4.8/5  (42)
(42)
How many moles of methanol, CH3OH, dissolved in 450.0 g of water are needed to make a 2.500 m solution?
(Short Answer)
4.9/5  (29)
(29)
Predict the deviation from Raoult's law when two liquids are mixed and the heat of the solution is large and the reaction exothermic.
(Multiple Choice)
4.8/5  (44)
(44)
A 5.00-g sample of a compound is dissolved in enough water to form 100.0 mL of solution. This solution has an osmotic pressure of 25 torr at 25°C. If it is assumed that each molecule of the solute dissociates into two particles (in this solvent), what is the molar mass of this solute?
(Multiple Choice)
4.9/5  (38)
(38)
A 5.96-g sample of a compound is dissolved in 222.0 g of benzene. The freezing point of this solution is 1.06°C below that of pure benzene. What is the molar mass of this compound? (Note: Kf for benzene = 5.12°C/m.)
(Multiple Choice)
5.0/5  (39)
(39)
When 100.0 g of a compound is dissolved in 1500. g of water, the freezing point of the solution is lowered to -1.35°C. Determine the molar mass of the compound.
(Short Answer)
4.8/5  (40)
(40)
Showing 61 - 80 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)