Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Based on intermolecular forces, which organic compound should have the highest boiling point?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
Of the three cubic unit cells, which lattice packing leaves the least amount of free space in the cell?
Free
(Multiple Choice)
4.9/5  (44)
(44)
Correct Answer:
D
The structure for CaF2 can be described as a face-centered array of Ca2+ ions with F- ions in the tetrahedral holes. The edge length of the unit cell is 5.45 *10-8 cm.
A) What fraction of the tetrahedral holes must be occupied by F- ions?
B) Calculate the density of CaF2.
C) If the ionic radius of F- is 1.36 * 10-8 cm, estimate the ionic radius of Ca2+. Tetrahedral holes are located along the body diagonals of each unit cell so that 1/4 (body diagonal) = rCa2+ + rF-.
Free
(Short Answer)
4.8/5  (35)
(35)
Correct Answer:
A) All tetrahedral holes are filled for the required 2:1 stoichiometry.
B) 3.20 g/cm3
C) 9.9 * 10-9 cm
Which of the following is the correct order of boiling points for NaNO3, C2H5OH, C2H6, and Ne?
(Multiple Choice)
4.9/5  (30)
(30)
When 1.00 mol of a pure liquid is vaporized at a constant pressure of 1.06 atm and at its boiling point of 313.4 K, 30.00 kJ of energy (heat) is absorbed and the volume change is +25.80 L. What is H for this process? (1 L-atm = 101.3 J)
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is most likely to be a gas at room temperature?
(Multiple Choice)
4.9/5  (42)
(42)
Which substance can be described as cations bonded together by mobile electrons?
(Multiple Choice)
4.8/5  (38)
(38)
Which substance involves no intermolecular forces except London dispersion forces?
(Multiple Choice)
4.7/5  (41)
(41)
In cubic closest-packed solids, what percentage of space is occupied by the spheres?
(Multiple Choice)
4.8/5  (31)
(31)
The resistance of a liquid to an increase in its surface area is called
(Multiple Choice)
4.7/5  (37)
(37)
Properties of liquids lie (closer to/further from) properties of a solid than to (or from) properties of a gas.
(Multiple Choice)
4.8/5  (40)
(40)
A certain substance has the phase diagram shown below. At which of the following values of T and P is the substance a pure liquid? 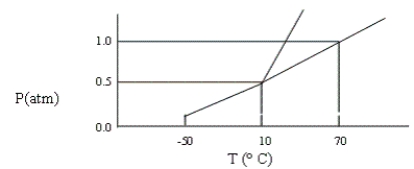
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following processes must exist in equilibrium with the condensation process when a measurement of vapor pressure is made?
(Multiple Choice)
4.8/5  (48)
(48)
When 1.00 mol of a pure liquid is vaporized at a constant pressure of 1.00 atm and at its boiling point of 330.8 K, 31.86 kJ of energy (heat) is absorbed and the volume change is +25.32 L. What is E for this process? (1 L-atm = 101.3 J)
(Multiple Choice)
5.0/5  (38)
(38)
Sodium oxide (Na2O) crystallizes in a structure in which the O2- ions are in a face-centered cubic lattice and the Na+ ions are in tetrahedral holes. What is the number of Na+ ions in the unit cell?
(Multiple Choice)
4.9/5  (42)
(42)
A liquid placed in a closed container will evaporate until equilibrium is reached. At equilibrium, which of the following statements is not true?
(Multiple Choice)
4.9/5  (46)
(46)
Chromium metal crystallizes as a body-centered cubic lattice. If the atomic radius of Cr is 1.25 angstroms, what is the density of Cr metal in grams per cubic centimeter?
(Multiple Choice)
4.9/5  (33)
(33)
The molar volume of a certain form of solid lead is 18 cm3/mol. Assuming cubic closest-packed structure, determine the following.
-The radius of a Pb atom
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)