Exam 17: Properties of Solutions
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Predict the deviation from Raoult's law when two liquids are mixed and the heat of the solution is small.
(Multiple Choice)
4.9/5  (34)
(34)
You have a 10.40-g mixture of table sugar (C12H22O11) and table salt (NaCl). When this mixture is dissolved in 150. g of water, the freezing point is found to be -2.24°C. Calculate the percent by mass of sugar in the original mixture. (Assume that the NaCl is completely dissociated.)
(Multiple Choice)
4.9/5  (35)
(35)
Using the data below, calculate the vapor pressure of benzene over a chloroform-benzene solution at 25°C, which contains 50.0 g of CHCl3 and 50.0 g of C6H6. Assume that the solution behaves ideally. 
(Multiple Choice)
4.8/5  (37)
(37)
You use 2.0 g of solid MX to prepare a saturated solution in 250. g of water. You find the freezing point to be -0.028°C. Calculate Ksp for the solid.
(Multiple Choice)
4.8/5  (41)
(41)
Consider a solution containing liquids A and B where the mole fraction of B is 0.60. Assuming ideality, calculate the mole fractions of A and B in the vapor at equilibrium with this solution at 25°C. (The vapor pressures of pure liquid A and pure liquid B at 25°C are 200. torr and 400. torr, respectively.)
(Essay)
4.7/5  (36)
(36)
A liquid-liquid solution is called an ideal solution if
I. it obeys PV = nRT.
II. it obeys Raoult's law.
III. solute-solute, solvent-solvent, and solute-solvent interactions are very similar.
IV. solute-solute, solvent-solvent, and solute-solvent interactions are quite different.
(Multiple Choice)
4.9/5  (37)
(37)
Liquid A has vapor pressure x. Liquid B has vapor pressure y, and x > y. What is the mole fraction of A in the liquid mixture if the vapor above the solution is 50% A?
(Multiple Choice)
4.8/5  (28)
(28)
Acetone (mw = 58.08, P 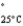 =232 mmHg) and butanone (mw = 72.11, P
=232 mmHg) and butanone (mw = 72.11, P 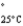 =100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
=100 mmHg) have the indicated molar masses and vapor pressures. A container holds 1.00 kg of butanone. How much acetone must be added to the butanone to elevate the total vapor pressure over the mixture to 125 mmHg at 25°C?
(Multiple Choice)
4.8/5  (40)
(40)
A solution contains 1 mol of liquid A and 3 mol of liquid B. The vapor pressure of this solution is 314 torr at 25°C. At 25°C, the vapor pressure of liquid A is 265 torr and the vapor pressure of liquid B is 355 torr. Which of the following is true?
(Multiple Choice)
4.8/5  (39)
(39)
How many milliliters of 11.8 M HNO3 are needed to prepare 386.2 mL of 0.18 M HNO3?
(Multiple Choice)
4.9/5  (49)
(49)
Diagram and label a vapor pressure diagram for an ideal solution of two volatile liquids. Indicate the deviation predicted by an endothermic heat of solution.
(Short Answer)
4.8/5  (42)
(42)
The freezing-point depression of a 3.00 × 10-3 M solution of a weak acid HA is 6.67 × 10-3 °C in water. Assuming that the molarity concentration and the molality concentration are the same and assuming ideal behavior, calculate Ka for the weak acid. Kf(H2O) = 1.86 °C kg/mol.
(Short Answer)
4.8/5  (36)
(36)
The molal freezing-point depression constants for benzene and water are 5.12 and 1.86, respectively. When 4.6 g of formic acid (HCOOH) is dissolved in 1.0 kg of benzene, the freezing point is depressed by 0.26°C. When the same amount of formic acid is dissolved in 1.0 kg of water, the freezing point is lowered by 0.19°C. To explain these results, we must assume that
(Multiple Choice)
4.9/5  (33)
(33)
A solution is made of 175.0 g of methanol (CH3OH) in 169.0 g of water. What is the mole fraction of methanol?
(Multiple Choice)
4.9/5  (39)
(39)
A salt solution sits in an open beaker. Assuming constant temperature and pressure, the vapor pressure of the solution
(Multiple Choice)
4.9/5  (28)
(28)
A 5.00-g sample of a compound is dissolved in enough water to form 100.0 mL of solution. This solution has an osmotic pressure of 25 torr at 25°C. If it is assumed that each molecule of the solute dissociates into two particles (in this solvent), what is the molar mass of this solute?
(Multiple Choice)
4.7/5  (46)
(46)
What is the molality of a solution of 31.8 g of ethanol (CH3CH2OH) in 405 mL of water if the density of water is 1.0 g/mL?
(Multiple Choice)
4.7/5  (38)
(38)
We can predict the solubility of a compound by looking at the sign of the enthalpy of solution.
(True/False)
4.8/5  (37)
(37)
The osmotic pressure of a solution saturated with a salt M5X3 is 1.96 × 10-2 atm at 25° C. Calculate Ksp for M5X3, assuming ideal behavior.
(Short Answer)
4.9/5  (38)
(38)
Showing 41 - 60 of 105
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)