Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Which one of the following is the strongest intermolecular force experienced by noble gases?
(Multiple Choice)
4.9/5  (30)
(30)
A material is made from Al, Ga, and As. The mole fractions of these elements are 0.25, 0.26, and 0.49, respectively. This material would be
(Multiple Choice)
4.8/5  (33)
(33)
The molar volume of a certain form of solid lead is 18 cm3/mol. Assuming cubic closest-packed structure, determine the following.
-The radius of a Pb atom
(Multiple Choice)
4.9/5  (39)
(39)
Below is a phase diagram for compound X. What is the normal boiling point of X is most likely to be? 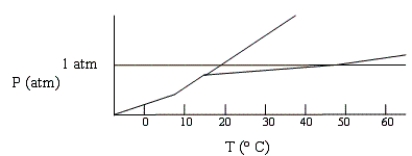
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following compounds has the lowest boiling point?
(Multiple Choice)
4.8/5  (40)
(40)
Which substance involves no intermolecular forces except London dispersion forces?
(Multiple Choice)
4.7/5  (40)
(40)
Consider an ionic compound CxAy where the anions (A) are in a body-centered cubic arrangement and the cations (C) are located in all of the faces of the cubic unit cell.
A. What is the empirical formula of the salt?
B. If the edge length of the unit cell is 5.00 × 10 cm and the molar masses of C and A are 50.0 g/mol and 100.0 g/mol, respectively, calculate the density of CxAy.
C. If the ionic radius of A is 200. pm, estimate the ionic radius of C.
(Short Answer)
4.7/5  (28)
(28)
Doping Se with As would produce a(n) __________ semiconductor with __________ conductivity compared to pure Se.
(Multiple Choice)
4.8/5  (44)
(44)
Make a sketch to show the hydrogen bonding between two acetic acid molecules (HC2H3O2).
(Essay)
4.8/5  (33)
(33)
A(n) _____ is a computer-controlled instrument used for carrying out the X-ray analysis of crystals.
(Multiple Choice)
4.9/5  (43)
(43)
100. g of ice at 0°C is added to 300.0 g of water at 60°C. Assuming no transfer of heat to the surroundings, what is the temperature of the liquid water after all the ice has melted?
(Short Answer)
4.8/5  (33)
(33)
Which of the following substances would you expect to have the lowest boiling point?
(Multiple Choice)
4.9/5  (25)
(25)
Which of the following processes must exist in equilibrium with the condensation process when a measurement of vapor pressure is made?
(Multiple Choice)
4.9/5  (44)
(44)
Identify the true statement(s) of the different types of holes in closest packed structures.
1. Trigonal holes are formed by three spheres in the same layer.
2. Tetrahedral holes are formed when a sphere sits in the dimple of four spheres in an adjacent layer.
3. Octahedral holes are formed between two sets of three spheres in adjoining layers of the closest packed structures.
(Multiple Choice)
4.9/5  (37)
(37)
Based on intermolecular forces, which organic compound should have the highest boiling point?
(Multiple Choice)
4.7/5  (31)
(31)
Showing 61 - 80 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)