Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
The molar volume of a certain form of solid lead is 18 cm3/mol. Assuming cubic closest-packed structure, determine the following.
-The number of Pb atoms per unit cell
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following chemical species has the highest boiling point?
(Multiple Choice)
4.8/5  (34)
(34)
You are given the following boiling-point data:  Which one of these liquids would you expect to have the highest vapor pressure at room temperature?
Which one of these liquids would you expect to have the highest vapor pressure at room temperature?
(Multiple Choice)
4.9/5  (38)
(38)
Aluminum metal crystallizes in a face-centered cubic structure. What is the relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E)?
(Multiple Choice)
4.8/5  (42)
(42)
On the basis of your knowledge of bonding in liquids and solids, which of the following substances has the highest melting temperature?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the compounds below is an example of a network solid?
(Multiple Choice)
4.9/5  (34)
(34)
A certain substance has the phase diagram shown below. At which of the following values of T and P is the substance a pure liquid? 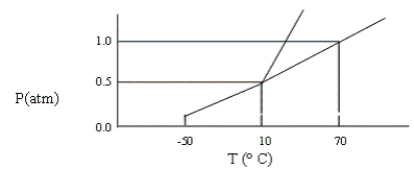
(Multiple Choice)
5.0/5  (36)
(36)
The structure for CaF2 can be described as a face-centered array of Ca2+ ions with F- ions in the tetrahedral holes. The edge length of the unit cell is 5.45 × 10-8 cm.A. What fraction of the tetrahedral holes must be occupied by F- ions?
B. Calculate the density of CaF2.C. If the ionic radius of F- is 1.36 × 10-8 cm, estimate the ionic radius of Ca2+. Tetrahedral holes are located along the body diagonals of each unit cell so that 1/4 (body diagonal) = rCa2+ + rF-.
(Essay)
4.9/5  (43)
(43)
Sodium oxide (Na2O) crystallizes in a structure in which the O2- ions are in a face-centered cubic lattice and the Na+ ions are in tetrahedral holes. What is the number of Na+ ions in the unit cell?
(Multiple Choice)
4.8/5  (43)
(43)
Based on intermolecular forces, which of the following will have the highest boiling point?
(Multiple Choice)
4.7/5  (30)
(30)
Which substance can be described as cations bonded together by mobile electrons?
(Multiple Choice)
4.8/5  (25)
(25)
The unit cell in a certain lattice consists of a cube formed by an anion at each corner, an anion in the center, and a cation at the center of each face. The unit cell contains a net
(Multiple Choice)
4.8/5  (46)
(46)
A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C, and the pressure is increased to 8 atm. Based on the phase diagram below, what will happen? 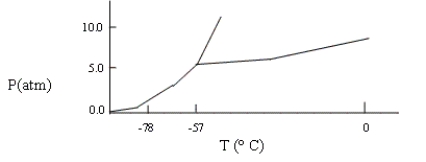
(Multiple Choice)
4.8/5  (45)
(45)
A sample of Co crystallizes in the hexagonal closest-packed (hcp) structure. Each atom in a layer is surrounded by and touches 6 other Co atoms. If the distance between Co atom centers within each layer is 2.0 × 102 pm, what is the distance between centers of nearest neighbors in adjacent layers?
(Multiple Choice)
4.9/5  (34)
(34)
When 1.00 mol of a pure liquid is vaporized at a constant pressure of 1.06 atm and at its boiling point of 332.6 K, 32.16 kJ of energy (heat) is absorbed and the volume change is 27.44 L. What is ΔH for this process? (1 L-atm = 101.3 J)
(Multiple Choice)
4.8/5  (35)
(35)
A certain metal fluoride crystallizes in such a way that the fluoride ions occupy simple cubic lattice sites, while the metal atoms occupy the body centers of half the cubes. What is the formula for the metal fluoride?
(Multiple Choice)
4.8/5  (33)
(33)
In the unit cell of sphalerite, Zn2+ ions occupy half the tetrahedral holes in a face-centered cubic lattice of S2- ions. What is the number of formula units of ZnS in the unit cell?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 21 - 40 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)