Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
ΔHvap for water is 40.7 kJ/mol. Calculate the boiling point of water at 0.500 atm.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is the smallest hole in a closest-packed lattice of spheres?
(Multiple Choice)
5.0/5  (36)
(36)
How much energy is needed to convert 60.6 grams of ice at 0.00°C to water at 55.4°C?
Specific heat of ice = 2.10 J/(g°C)
Specific heat of water = 4.18 J/(g°C)
Heat of fusion = 333 J/g
Heat of vaporization = 2258 J/g
(Multiple Choice)
4.9/5  (38)
(38)
What is the radius of the largest sphere that can be placed at the center of a face-centered cubic unit cell of a cubic closest-packed array of spheres if the spheres have diameters of 4.00 × 102 pm?
(Multiple Choice)
4.8/5  (36)
(36)
You are given a small bar of an unknown metal, X. You find the density of the metal to be 10.5 g/cm3. An X-ray diffraction experiment measures the edge of the unit cell as 409 pm. Assuming that the metal crystallizes in a face-centered cubic lattice, what is X most likely to be?
(Multiple Choice)
4.9/5  (35)
(35)
The heat of combustion of bituminous coal is 2.5 × 104 J/g. What quantity of the coal is required to produce the energy to convert 100. lb of ice at 0°C to steam at 100°C?
(Short Answer)
4.9/5  (37)
(37)
Which of the following statements is true about p-type silicon?
(Multiple Choice)
4.9/5  (36)
(36)
KCl crystallizes in a structure like NaCl. The ionic radius of Na+ is 0.525 times the ionic radius of Cl- and 0.714 times the ionic radius of K+.A. Estimate the ratio of the unit cell edge length for KCl to that for NaCl.B. Estimate the ratio of the density of NaCl to that of KCl.
(Short Answer)
4.9/5  (33)
(33)
A certain solid substance that is very hard, has a high melting point, and is nonconducting unless melted is most likely to be
(Multiple Choice)
4.8/5  (36)
(36)
The heat of vaporization of a certain refrigerant is 156 J/g. Calculate the number of kilograms of refrigerant that must be evaporated to freeze a tray of 17 one-ounce (1 oz = 28 g) ice cubes starting with the water at 13°C.Heat capacity (water) = 4.184 J/g °C
ΔHfusion (water) = 333 J/g
(Multiple Choice)
4.8/5  (28)
(28)
The normal boiling point of liquid X is less than that of Y, which is less than that of Z. Which of the following is the correct order of increasing vapor pressure of the three liquids at STP?
(Multiple Choice)
4.9/5  (34)
(34)
Pure rubidium crystallizes in a body-centered cubic lattice; the edge length of the unit cell is 562 pm. What is the density of rubidium in grams per cubic centimeter?
(Multiple Choice)
4.9/5  (44)
(44)
Metallic copper crystallizes in a face-centered cubic lattice. The volume of the unit cell is 4.11 × 108 pm3. What is the density of copper metal?
(Multiple Choice)
5.0/5  (41)
(41)
When 1.00 mol of a pure liquid is vaporized at a constant pressure of 1.00 atm and at its boiling point of 320.0 K, 28.80 kJ of energy (heat) is absorbed and the volume change is +24.90 L. What is ΔE for this process? (1 L-atm = 101.3 J)
(Multiple Choice)
4.8/5  (42)
(42)
Given the graph below, what is the boiling point of carbon tetrachloride at standard pressure? 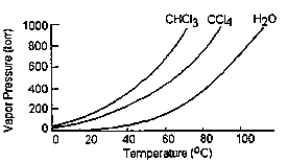
(Multiple Choice)
4.9/5  (27)
(27)
A salt, MY, crystallizes in a body-centered cubic structure with a Y- anion at each cube corner and an M+ cation at the cube center. Assuming that the Y- anions touch each other and the M+ cation at the center, and that the radius of Y- is 1.44 × 102 pm, what is the radius of M+?
(Multiple Choice)
4.7/5  (42)
(42)
Knowing that ΔHvap for water is 40.7 kJ/mol, calculate Pvap of water at 37°C.
(Multiple Choice)
4.8/5  (34)
(34)
The table below lists the ionic radii for the cations and anions in three ionic compounds.  Each compound has a cubic structure like NaCl, CsCl, or ZnS. Use the ratio of cation radius to anion radius to predict the structure formed (that of NaCl, CsCl, or ZnS). Then estimate the density of each compound. For ZnS structures, the holes occupied lie along the body diagonals of the unit cell so that 1/4 (body diagonal) = r+ + r-.
Each compound has a cubic structure like NaCl, CsCl, or ZnS. Use the ratio of cation radius to anion radius to predict the structure formed (that of NaCl, CsCl, or ZnS). Then estimate the density of each compound. For ZnS structures, the holes occupied lie along the body diagonals of the unit cell so that 1/4 (body diagonal) = r+ + r-.
(Essay)
4.8/5  (33)
(33)
Showing 41 - 60 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)