Exam 16: Liquids and Solids
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Shown below is a phase diagram for sulfur (not drawn to scale). Sulfur can exist in solid modifications, rhombic and monoclinic, denoted by SR and SM, respectively. Which of the following statements is incorrect? 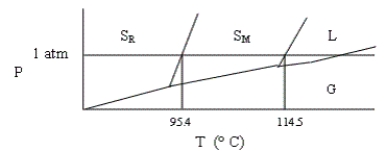
(Multiple Choice)
4.9/5  (35)
(35)
Alkali halides commonly have either the sodium chloride structure or the cesium chloride structure. The molar mass of CsCl is 2.88 times the molar mass of NaCl, and the edge length of the unit cell for NaCl is 1.37 times the edge length of the CsCl unit cell. Determine the ratio of the density of CsCl to the density of NaCl.
(Multiple Choice)
4.9/5  (35)
(35)
In cubic closest-packed solids, what percentage of space is occupied by the spheres?
(Multiple Choice)
4.9/5  (41)
(41)
Given the phase diagram shown below, which of the following statements is false? 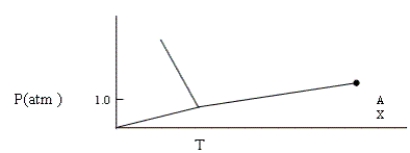
(Multiple Choice)
5.0/5  (37)
(37)
A certain substance, X, has a triple-point temperature of 20°C at a pressure of 2.0 atm. Which one of the following statements cannot possibly be true?
(Multiple Choice)
4.9/5  (30)
(30)
Shown below is a phase diagram for compound X. How will the melting point of X change with increased pressure? 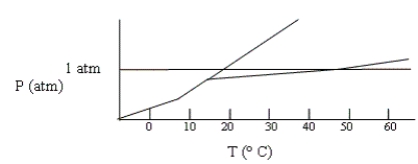
(Multiple Choice)
4.9/5  (27)
(27)
A liquid placed in a closed container will evaporate until equilibrium is reached. At equilibrium, which of the following statements is not true?
(Multiple Choice)
4.8/5  (38)
(38)
How many grams of ice would be melted by the energy obtained as 18.0 g of steam is condensed at 100°C and cooled to 0°C?
(Short Answer)
4.9/5  (32)
(32)
The resistance of a liquid to an increase in its surface area is called
(Multiple Choice)
4.8/5  (42)
(42)
A metal crystallizes in a body-centered unit cell with an edge length of 2.05 × 102 pm. Assume the atoms in the cell touch along the cube diagonal. What will be the percentage of empty volume in the unit cell?
(Multiple Choice)
4.7/5  (37)
(37)
Chromium metal crystallizes as a body-centered cubic lattice. If the atomic radius of Cr is 1.25 angstroms, what is the density of Cr metal in grams per cubic centimeter?
(Multiple Choice)
4.8/5  (29)
(29)
Mn crystallizes in the same cubic unit cell as Cu. Assuming that the radius of Mn is 5.6% larger than the radius of Cu and that the density of copper is 8.96 g/cm3, calculate the density of Mn.
(Multiple Choice)
4.9/5  (35)
(35)
Given below are the temperatures at which two different liquid compounds with the same empirical formula have a vapor pressure of 400 torr.  Which of the following statements is false?
Which of the following statements is false?
(Multiple Choice)
4.7/5  (36)
(36)
The unit cell in this two-dimensional crystal contains __________ Xs and __________ Os. 
(Multiple Choice)
4.8/5  (27)
(27)
Based on the phase diagram shown below, which of the following statements are correct?
I. Sublimation occurs at a point in the transformation that falls along a straight line from point A to point F.
II. C and E represent points where the gas and liquid phases are in equilibrium.
III. ΔHvap can be measured at point B.
IV. Molecules at point D have a greater average kinetic energy than those at point F.
V. The temperature at point E is called the critical temperature of the compound. 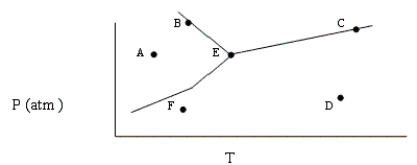
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following statements is true about the vapor pressures of methane (CH4) and ammonia (NH3)?
(Multiple Choice)
4.9/5  (48)
(48)
The triple point of iodine is at 90 torr and 115°C. This means that liquid I2
(Multiple Choice)
4.7/5  (39)
(39)
MnO has either a structure like NaCl or a structure like CsCl. The edge length of the MnO unit cell is 4.47 × 10-8 cm, and the density of MnO is 5.28 g/cm3.
A. Does MnO crystallize like NaCl or like CsCl?
B. If the ionic radius of oxygen is 140. pm, estimate the ionic radius of manganese.
C. Does the calculated ratio of the cation radius to the anion radius for MnO support your answer in part A? Explain.
(Short Answer)
4.8/5  (34)
(34)
Showing 81 - 100 of 111
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)