Exam 42: Nuclear Physics
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
The relationship between the activity R, the disintegration constant λ, and the remaining number N of radioactive nuclei is:
(Multiple Choice)
4.9/5  (34)
(34)
The model of the nucleus in which each nucleon has its own well-defined quantum numbers is called the:
(Multiple Choice)
4.9/5  (33)
(33)
The smallest particle of any chemical element that can exist by itself and yet retain the qualities that distinguish it as that element is:
(Multiple Choice)
4.9/5  (35)
(35)
Magnesium has atomic number 12, hydrogen has atomic number 1, and helium has atomic number 2. In the nuclear reaction 24Mg + 2H ( ) + 4He the missing quantity is:
(Multiple Choice)
4.7/5  (30)
(30)
A certain nucleus, after absorbing a neutron, emits a - and then splits into two alpha particles. The (A, Z) of the original nucleus must have been:
(Multiple Choice)
4.8/5  (31)
(31)
Starting with a sample of pure 66Cu, 7/8 of it decays into Zn in 15 minutes. The corresponding half-life is:
(Multiple Choice)
4.9/5  (34)
(34)
A nucleus with mass number A and atomic number Z undergoes - decay. The mass number and atomic number, respectively, of the daughter nucleus are:
(Multiple Choice)
4.8/5  (33)
(33)
The half-life of a radioactive isotope is 140 days. In how many days does the decay rate of a sample of this isotope decrease to one fourth its initial decay rate?
(Multiple Choice)
4.9/5  (40)
(40)
A radioactive atom X emits a - particle. The resulting atom:
(Multiple Choice)
4.9/5  (32)
(32)
The half-life of a radioactive isotope is 6.5 h. If there are initially 48 * 1032 atoms of this isotope, the number of atoms of this isotope remaining after 26 h is:
(Multiple Choice)
4.9/5  (41)
(41)
Iron has atomic number 26. Naturally mined iron contains isotopes of mass numbers 54, 56, 57, and 58. Which of the following statements is FALSE?
(Multiple Choice)
4.8/5  (36)
(36)
Bromine, with atomic mass 79.942 u, is composed of nearly equal amounts of two isotopes, one of which contains 79 nucleons per atom. The mass number of the other isotope is:
(Multiple Choice)
4.9/5  (29)
(29)
Radioactive element A decays to the stable element B with a half-life T. Starting with a sample of pure A and no B, which graph below most correctly shows the number of A atoms, NA, as a function of time t? 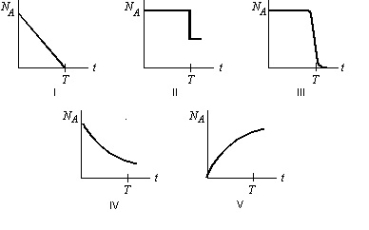
(Multiple Choice)
4.8/5  (41)
(41)
The 66Cu (Z = 29) produced in a nuclear bombardment is unstable, changing to 66Zn (Z = 30) by the emission of:
(Multiple Choice)
4.7/5  (30)
(30)
Showing 41 - 60 of 68
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)