Exam 13: When Reactants Turn Into Products
Exam 1: What Is Chemistry102 Questions
Exam 2: The Numerical Side of Chemistry201 Questions
Exam 3: The Evolution of Atomic Theory88 Questions
Exam 4: The Modern Model of the Atom144 Questions
Exam 5: Chemical Bonding and Nomenclature230 Questions
Exam 6: The Shape of Molecules138 Questions
Exam 7: Intermolecular Forces and the Phases of Matter107 Questions
Exam 8: Chemical Reactions122 Questions
Exam 9: Stoichiometry and the Mole241 Questions
Exam 10: Electron Transfer in Chemical Reactions138 Questions
Exam 11: What If There Were No Intermolecular Forces - the Ideal Gas118 Questions
Exam 12: Solutions143 Questions
Exam 13: When Reactants Turn Into Products106 Questions
Exam 14: Chemical Equilibrium135 Questions
Exam 15: Electrolytes, Acids, and Bases153 Questions
Exam 16: Nuclear Chemistry117 Questions
Exam 17: The Chemistry of Carbon105 Questions
Exam 18: Synthetic and Biological Polymers60 Questions
Select questions type
Which of the following factors could increase the reaction rate? 2A + B2 → 2AB
(I) decreasing the temperature
(II) adding a catalyst
(III) increasing the surface area of solid reactants
(Multiple Choice)
4.7/5  (29)
(29)
Calculate a value for ΔErxn for a chemical reaction if the reactants have an energy of -400 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?
(Short Answer)
4.8/5  (40)
(40)
In each of the following diagrams, the x-axis is the reaction coordinate and the y-axis is the potential energy. Which diagram corresponds to a reaction that has an activation energy of 35 kJ and an overall reaction energy of -110 kJ?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following responses contains the two rate factors that comprise the rate constant (k) for a given chemical reaction?
(Multiple Choice)
4.9/5  (36)
(36)
The following data was obtained at 100 °C. 2Y (g) + X (g) → Z (g) 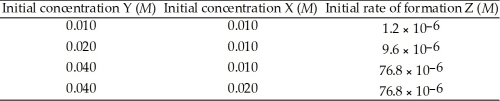 The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
4.8/5  (45)
(45)
The following data was obtained at 20 °C. 2Y (g) → Z (g)  The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following is not true when the temperature of the reaction mixture is decreased?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following is not a term involved in determining the overall rate of a chemical reaction?
(Multiple Choice)
4.9/5  (29)
(29)
The value of Ea(reaction) is equal to the difference Ea(reactants) - Ea(products).
(True/False)
4.8/5  (37)
(37)
If the concentration of A is doubled while the concentration of B is unchanged, the rate will ________. A (g) + 3 B (g) → C (g) + 2 D (g)
Rate = k[A][B]3
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following "adjustments" can be made to a chemical reaction system to increase the rate of reaction?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following reaction coordinate diagrams represents a chemical reaction that is exothermic?
(Multiple Choice)
4.8/5  (41)
(41)
The reactants must collide with an energy greater than the energy of activation for the reaction to occur.
(True/False)
4.8/5  (37)
(37)
Match the rate of each reaction with the overall order .
-Rate = k[A][B]-1
(Multiple Choice)
4.7/5  (38)
(38)
The reaction: HCl + NaOH → H₂O + NaCl is a substitution reaction.
(True/False)
4.8/5  (47)
(47)
Which of the following is true about the activation energy?
(Multiple Choice)
4.9/5  (46)
(46)
The fraction of collisions with the proper orientation depends on ________.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 21 - 40 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)