Exam 13: When Reactants Turn Into Products
Exam 1: What Is Chemistry102 Questions
Exam 2: The Numerical Side of Chemistry201 Questions
Exam 3: The Evolution of Atomic Theory88 Questions
Exam 4: The Modern Model of the Atom144 Questions
Exam 5: Chemical Bonding and Nomenclature230 Questions
Exam 6: The Shape of Molecules138 Questions
Exam 7: Intermolecular Forces and the Phases of Matter107 Questions
Exam 8: Chemical Reactions122 Questions
Exam 9: Stoichiometry and the Mole241 Questions
Exam 10: Electron Transfer in Chemical Reactions138 Questions
Exam 11: What If There Were No Intermolecular Forces - the Ideal Gas118 Questions
Exam 12: Solutions143 Questions
Exam 13: When Reactants Turn Into Products106 Questions
Exam 14: Chemical Equilibrium135 Questions
Exam 15: Electrolytes, Acids, and Bases153 Questions
Exam 16: Nuclear Chemistry117 Questions
Exam 17: The Chemistry of Carbon105 Questions
Exam 18: Synthetic and Biological Polymers60 Questions
Select questions type
The rate is second order with respect to the reactant and second order overall.
Rate = k[A]2
(True/False)
4.9/5  (35)
(35)
The following data was obtained at 20 °C. X (g) → Y (g)  The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
5.0/5  (47)
(47)
The following data was obtained at 20 °C. 2W (g) → V (g) 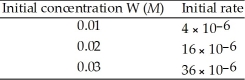 The rate law for this reaction is ________.
The rate law for this reaction is ________.
(Multiple Choice)
4.9/5  (28)
(28)
The greater the concentration of the reactants, the greater the rate of the reaction.
(True/False)
4.8/5  (36)
(36)
Knowing the mechanism of a reaction is advantageous because one may influence its rate to make the reaction useful.
(True/False)
4.8/5  (33)
(33)
Reaction rates can be varied by changing either the concentrations of the reacting species or the temperature at which the reaction is carried out.
(True/False)
4.9/5  (41)
(41)
Consider the reaction A → B with the following data: Energy of reactants = 50 kJ/mole;
Energy of products = 70 kJ/mole;
Energy of transition state = 80 kJ/mole.
The reaction is ________, has a heat of reaction of ________ kJ/mole, and the energy of activation is ________ kJ/mole.
(Multiple Choice)
4.7/5  (43)
(43)
If the reaction has a negative overall energy change, then ________.
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following represents a reaction coordinate diagram for a chemical reaction whose ΔErxn = 0?
(Multiple Choice)
4.8/5  (42)
(42)
Match the rate of each reaction with the overall order .
-Rate = k[A]2[B]-1
(Multiple Choice)
4.7/5  (28)
(28)
Based on the collision theory, decreasing temperature for a reaction increases the reaction rate.
(True/False)
4.7/5  (37)
(37)
Examine this reaction coordinate diagram, and answer the questions that follow. 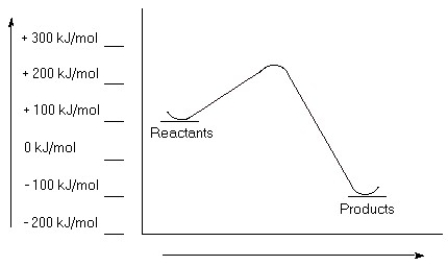 a) Estimate the value for the activation energy for this reaction.
b) Calculate ΔErxn.
c) Is this reaction exothermic or endothermic?
a) Estimate the value for the activation energy for this reaction.
b) Calculate ΔErxn.
c) Is this reaction exothermic or endothermic?
(Essay)
4.9/5  (41)
(41)
Consider the reaction X → Y with the following data: Energy of reactants = 25 kJ/mole;
Energy of products = 75 kJ/mole;
Energy of transition state = 100 kJ/mole.
The energy of activation for the reverse reaction is ________ kJ/mole.
(Multiple Choice)
4.8/5  (39)
(39)
The following data was obtained at 100 °C. 2 Y (g) + X (g) → Z (g) 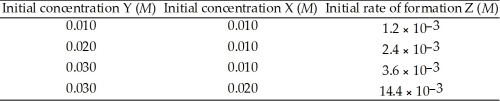 The rate law for this reaction is ________..
The rate law for this reaction is ________..
(Multiple Choice)
4.9/5  (32)
(32)
The order of the reaction with respect to A, B, and the total order respectively is ________. A + B → Products
Rate = k[A]3[B]0
(Multiple Choice)
4.7/5  (25)
(25)
Which of the following statements regarding reaction mechanisms is true?
(Multiple Choice)
4.9/5  (38)
(38)
In a catalyzed reaction the activation energy is ________ when compared to the uncatalyzed reaction.
(Multiple Choice)
4.7/5  (37)
(37)
Showing 81 - 100 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)