Exam 9: Addition Reactions of Alkenes
Exam 1: A Review of General Chemistry: Electrons, Bonds, and Molecular Properties191 Questions
Exam 2: Molecular Representations154 Questions
Exam 3: Acids and Bases126 Questions
Exam 4: Alkanes and Cycloalkanes114 Questions
Exam 5: Stereoisomerism125 Questions
Exam 6: Chemical Reactivity and Mechanisms110 Questions
Exam 7: Substitution Reactions123 Questions
Exam 8: Alkenes: Structure and Preparation Via Elimination Reactions111 Questions
Exam 9: Addition Reactions of Alkenes148 Questions
Exam 10: Alkynes166 Questions
Exam 11: Radical Reactions90 Questions
Exam 12: Synthesis95 Questions
Exam 13: Alcohols and Phenols119 Questions
Exam 14: Ethers and Epoxides; Thiols and Sulfides130 Questions
Exam 15: Infrared Spectroscopy and Mass Spectrometry129 Questions
Exam 16: Nuclear Magnetic Resonance Spectroscopy114 Questions
Exam 17: Conjugated Pi Systems and Pericyclic Reactions131 Questions
Exam 18: Aromatic Compounds98 Questions
Exam 19: Aromatic Substitution Reactions109 Questions
Exam 20: Aldehydes and Ketones143 Questions
Exam 21: Carboxylic Acids and Their Derivatives117 Questions
Exam 22: Alpha Carbon Chemistry: Enols and Enolates131 Questions
Exam 23: Amines97 Questions
Exam 24: Carbohydrates122 Questions
Exam 25: Amino Acids, Peptides, and Proteins115 Questions
Exam 26: Lipids102 Questions
Exam 27: Synthetic Polymers100 Questions
Select questions type
Identify the structure(s) of the expected major organic product(s) of the following reaction. Draw the products in the expected chair conformations and include regiochemical and/or stereochemical details as needed. 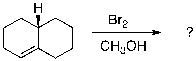
(Essay)
4.8/5  (38)
(38)
Provide the structure(s) of the expected major organic product(s) generated upon completion of the following reaction scheme: 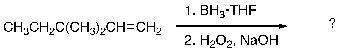
(Essay)
4.9/5  (33)
(33)
For the complete reduction of 1 mole of (3E,5E)-hepta-1,3,5-triene with H2 using a Pd catalyst, how many moles of H2 are consumed?
(Multiple Choice)
4.9/5  (39)
(39)
The rule that correctly predicts the regiochemistry of most ionic additions to alkenes is named after which chemist?
(Short Answer)
4.7/5  (43)
(43)
Treatment of 1,2-dimethylcyclopentene with OsO4, followed by aqueous NaHSO3, produces which of the following: 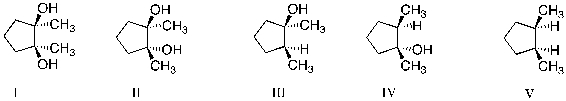
(Multiple Choice)
4.9/5  (42)
(42)
What is the expected major product for the following reaction? 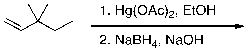
(Multiple Choice)
4.8/5  (40)
(40)
The regioselectivity and stereospecificity in hydrohalogenation of an alkene is best described as:
(Multiple Choice)
4.9/5  (35)
(35)
What is the major product for the following reaction sequence? 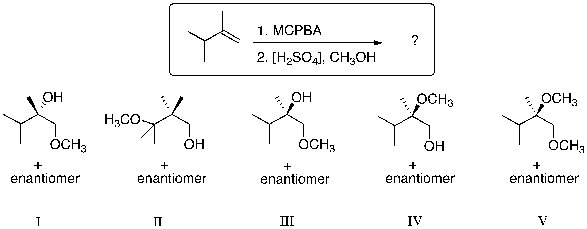
(Multiple Choice)
4.9/5  (33)
(33)
Wilkinson's catalyst accomplishes which of the listed molecular transformations?
(Multiple Choice)
4.8/5  (37)
(37)
What are the major products for the following reaction sequence? 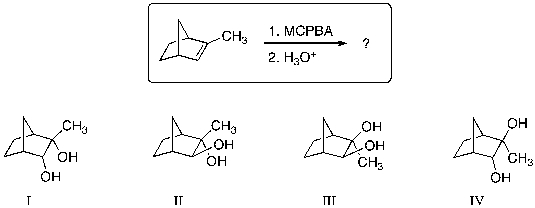
(Multiple Choice)
4.8/5  (32)
(32)
Which of the structures shown depicts the most stable carbocation intermediate formed in the hydrohalogenation reaction shown? 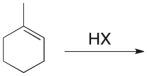
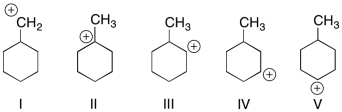
(Multiple Choice)
4.9/5  (34)
(34)
Provide the structure(s) of the expected major organic product(s) generated upon completion of the following reaction scheme: 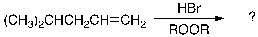
(Essay)
4.8/5  (40)
(40)
What major product is expected from the following reaction? 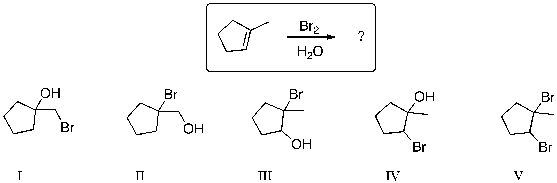
(Multiple Choice)
4.8/5  (34)
(34)
What is the expected product for the hydrogenation of an alkene?
(Multiple Choice)
4.9/5  (40)
(40)
How many carbonyl groups are generated upon treatment of the molecule below with ozone, followed by zinc metal and water? 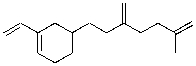
(Multiple Choice)
4.9/5  (33)
(33)
For the reaction sequence below, identify the expected major products. 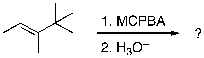
(Multiple Choice)
4.7/5  (39)
(39)
How many moles of hydrogen (H2) are required to completely reduce 1 mole of cis-2,3,3-trimethylhepta-1,5-diene?
(Multiple Choice)
4.8/5  (36)
(36)
Which reagents are most likely to accomplish the transformation shown below? 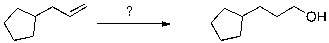
(Multiple Choice)
4.9/5  (37)
(37)
Starting with (2R,3R)-2-chloro-3,4-dimethylpentane, a) draw the expected major product for each step, including regiochemical and stereochemical details when needed. In addition, b) briefly explain the mechanistic rationale for each transformation.
i. t-BuOK/t-BuOH, heat
ii. MCPBA/CH2Cl2
iii. H3O+
(Essay)
4.8/5  (32)
(32)
Which of the reagents below are expected to convert cyclopentene into cyclopentane?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 121 - 140 of 148
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)